Abstract
The current study was designed to examine the effects of intracerebroventricular injections of SHU9119 [a nonselective melanocortin receptor (McR) antagonist] and MCL0020 (a selective McR antagonist) on the serotonin-induced eating and drinking responses of broiler cockerels deprived of food for 24 h (FD24). For Experiment 1, the chickens were intracerebroventricularly injected with 2.5, 5, and 10 µg serotonin. In Experiment 2, the chickens received 2 nmol SHU9119 before being injected with 10 µg serotonin. For Experiment 3, the chickens were given 10 µg serotonin after receiving 2 nmol MCL0020, and the level of food and water intake was determined 3 h post-injection. Results of this study showed that serotonin decreased food intake but increased water intake among the FD24 broiler cockerels and that these effects occurred in a dose-dependent manner. The inhibitory effect of serotonin on food intake was significantly attenuated by pretreatment with SHU9119 and MCL0020. However, the stimulatory effect of serotonin on water intake was not altered by this pretreatment. These results suggest that serotonin hypophagia and hyperdipsia were mediated by different mechanisms in the central nervous system, and that serotonin required downstream activation of McRs to promote hypophagia but not hyperdipsia in the FD24 chickens.
Keywords: chicken, food and water intake, MCL0020, serotonin, SHU9119
Introduction
Over the past decades, many studies have investigated brain neurochemical systems that regulate food and water intake in animals. From these, a number of major central neurotransmitter circuits that appear to contribute to these behaviors have been identified. Catecholaminergic and serotonergic systems are amongst the most extensively investigated [15]. However, information for avian species is scarce and controversial. The effect of serotonin central injection has been studied on a limited scale in avian species, but appears to depend on the lineages (breeding lines) examined. In Leghorn chickens and turkeys deprived of food for 24 h (FD24) or satiated, intracerebroventricular (ICV) injections of serotonin induce a significant decrease in food intake [2] while increasing water intake among sated chickens but decreasing water consumption among the fasted birds [4]. These data emphasize the need to evaluate differences associated with bird strains or feeding habits in studies of systems that regulate avian food intake.
Proopiomelanocortin (POMC) is a precursor for three major peptide families: the adrenocorticotropins, melanocortins and the β-endorphins. The α-melanocyte stimulating hormone (α-MSH) derived from a multifunctional POMC inhibits food intake in mammals. ICV administration of α-MSH or its potential agonist (MTII), suppresses food intake [5,19] while the ICV injection of a melanocortin antagonist, SHU9119 or HS014, stimulates feeding behavior in rats [5]. Furthermore, targeted disruption of melanocortin receptors (McRs) results in obesity, hyperphagia, and hyperinsulinemia [9]. ICV injection of α-MSH in neonatal fasting chickens leads to significantly decreased food intake [12]. These reports suggest that α-MSH is essential for regulating feeding behavior and bodyweight by reducing food intake in mammals and birds. However, the effects of α-MSH on food and water intake in avians have not been fully elucidated.
Based on findings in the literature and considering that serotonin and melanocortin have identical effects on feeding behavior in birds, we hypothesized that melanocortin mediates serotonin signaling in the avian hypothalamus. Therefore, the current study was designed to determine for the first time whether blocking melanocortin 3 and 4 receptors (Mc3-Rs and Mc4-Rs) affects serotonin-induced feeding and drinking responses in FD24 broiler cockerels. We also assessed whether Mc3-Rs and Mc4-Rs are necessary downstream targets for serotonin-induced food and water intake in fasted chickens.
Materials and Methods
Animals
Seventy two broiler cockerels (Eshragh, Iran) were reared in heated batteries with continuous lighting until 3 weeks of age. The chickens were provided a mash diet (21% protein and 2,869 kcal/kg of metabolizable energy; Pars animal Feed, Iran) and water ad libitum. At approximately 2 weeks of age, the chickens were transferred to metabolic cages that each contained one chicken. The room was maintained at a temperature of 22 ± 1℃ with 50% humidity, and continuously lighted [20]. Animal handling and experimental procedures were performed according to the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health (USA) and the current laws of the Iranian government.
Drugs
Serotonin, SHU9119 (a nonselective Mc3-R and Mc4-R antagonist) and MCL0020 (a selective Mc4-R antagonist) were purchased from Tocris Bioscience (Tocris, UK). All solutions were prepared in a 0.9% pyrogen-free NaCl solution (saline) that served as the vehicle control.
Surgical preparation
At 3 weeks of age, the broilers were anesthetized with xylazin [1 mg/kg body weight, intramuscular (IM) injection] and ketamine (30 mg/kg bodyweight, IM) [29]. A 23-gauge thin-walled stainless steel guide cannula (Razipakhsh, Iran) was then stereotaxically implanted into the right lateral ventricle using a technique previously described by Denbow et al. [3]. The stereotaxic specifications were anterior/posterior: 6.7 mm, lateral: 0.7 mm and horizontal: 3.5~4 mm below the dura mater with the head oriented as described by Van Tienhoven and Juhász [30]. The cannula was secured with three stainless steel screws placed into the calvaria surrounding each guide cannula. Acrylic dental cement (Pars Acryl, Iran) was then applied to the screws and guide cannula. An orthodontic No. 014 wire (American Orthodontics, USA) trimmed to the exact length of the guide cannula was inserted into the guide cannula when the chickens were not being used for the experiments. Lincospectin (Razak, Iran) was applied to the incision to prevent possible infections. The chickens were allowed a minimum of 5 days to recover prior to receiving injections of solutions.
Experimental procedures
To evaluate the possible involvement of central McRs in serotonin induction of eating responses, the effects of centrally administered SHU9119 and MCL0020 on serotonin-associated eating responses of the chickens were investigated. Injections were delivered with a 29-gauge, thin-walled stainless steel injecting cannula (Razipakhsh, Iran) that extended 1.0 mm beyond the guide cannula. This injecting cannula was connected through 60-cm polyethylene-20 tubing (Parsian tube, Iran) to a 10-µL Hamilton syringe (Hamilton, Switzerland). All drugs were injected over a period of 60 sec. The solution was then allowed to diffuse from the tip of the cannula into the ventricle for an additional 60 sec.
All experimental procedures were performed from 10:00 am to 4:00 pm. The chickens were removed from the cages, restrained by hand, and then returned to the cages after receiving the injection. The birds were handled and mock injected daily during the 5-day recovery period in order for them to adapt to the injection procedure. Twenty-four h before initiating the experiments, the animals were deprived of food but still had access to water ad libitum. Immediately after receiving the injections, the chickens were returned to their individual, computerized metabolic cages (Altromin, Germany) where food and water intake was automatically measured. Fresh food and water were given at the time of injection (or after the second injection for Experiments 2 and 3), and cumulative food (g) and water (mL) intake were recorded 30, 60, 90, 120, 150, and 180 min after the injection. Placement of the guide cannula into the ventricle was verified by the presence of cerebrospinal fluid and an ICV injection of 10 µL methylene blue followed by an analysis of frozen brain tissue sections performed at the end of the experiments.
Experiment 1 was designed to examine the effect of ICV injections of serotonin on the food and water intake of FD24 chickens (n = 6 per group). The chickens received 2.5, 5, and 10 µg serotonin in 10 µL of saline. The control group was injected with 10 µL of saline. For Experiment 2, each chicken received two injections. The first injection contained either 0 or 2 nmol SHU9119 in 5 µL of saline. The second injection consisted of either 0 or 10 µg serotonin in 5 µL saline delivered 15 min after the first injection as described in Table 1 (n = 6 per group). All injections for the control group contained only 5 µL of saline. Experiment 3 was conducted similar in a manner similar to Experiment 2 except that the chickens received 0 or 2 nmol MCL0020 instead of SHU9119. All animals in Experiments 1, 2, and 3 were deprived of food for 24 h prior to initiating the study. All doses of serotonin, SHU9119 and MCL0020 were calculated based on previous and pilot studies [4,24].
Table 1.
Treatment procedure for Experiment 2
S: saline, control, SHU: SHU9119.
Statistical analysis
Cumulative food intake was analyzed by a one-way analysis of variance (ANOVA) and is presented as the mean ± SE. For treatments found to have an effect according to the ANOVA, mean values were compared with post hoc Bonferroni and Dunnett tests. p-values ≤ 0.05 were considered to indicate significant differences between the treatments.
Results
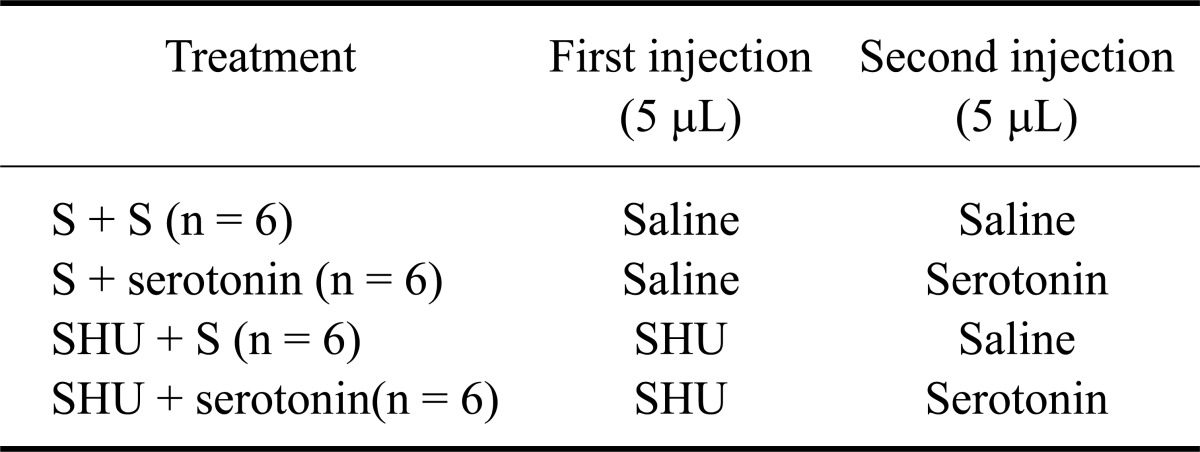
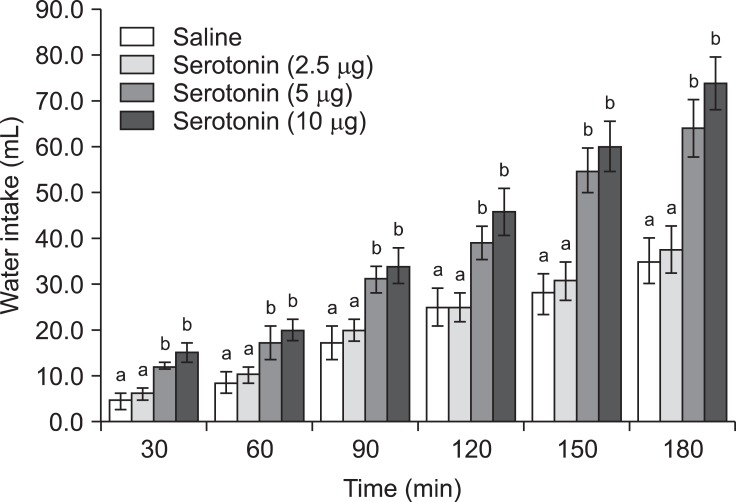
The feeding and drinking responses of the broiler chickens to ICV injections of serotonin, SHU9119, and MCL0020 are shown in Figs. 1~6. In Experiment 1, serotonin injected into the lateral ventricle of FD24 chickens caused a dose-dependent decrease in food consumption and increase in water intake compared to the control group [Figs. 1 and 2; p ≤ 0.05; f (3, 25) = 12.43 and f (3, 25) = 15.68, respectively]. Serotonin (5 and 10 µg doses) had significant anorexic and dipsogenic effects that lasted for at least 180 min. For the subsequent experiments, a 10-µg dose of serotonin was used because it was found to significantly decrease food consumption but increase water intakes in the FD24 birds without affecting other non-ingestive behavioral parameters.
Fig. 1.
Effect of intracerebroventricular (ICV) injection of serotonin at different doses on food intake in chickens deprived of food for 24 h (FD24). Data are presented as the mean ± SE. Lowercase letters (a, b, and c) indicate significant differences between the treatments (p ≤ 0.05).
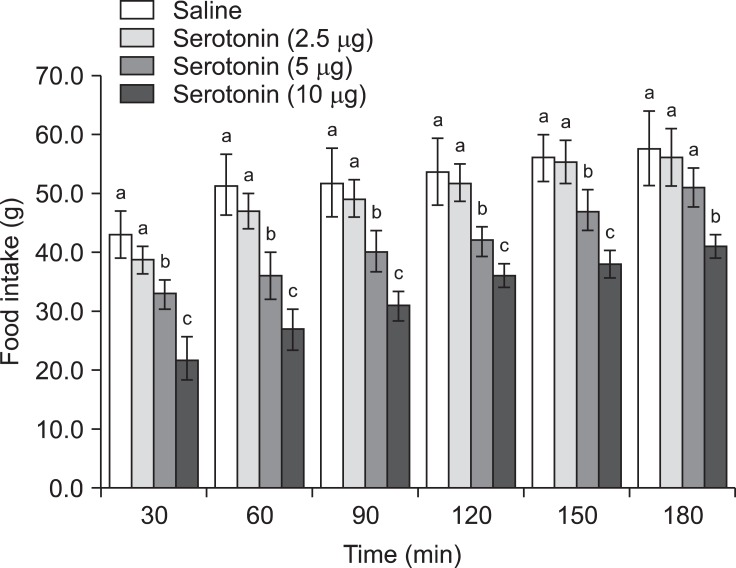
Fig. 6.
Effect of ICV delivery of MCL0020 (2 nmol) followed by serotonin (10 µg) on water intake in FD24 chickens. Data are presented as the mean ± SE. Lowercase letters (a and b) indicate significant differences between the treatments (p ≤ 0.05).
Fig. 2.
Effect of ICV injection of serotonin at different doses on water intake in FD24 chickens. Data are presented as the mean ± SE. Lowercase letters (a and b) indicate significant differences between the treatments (p ≤ 0.05).
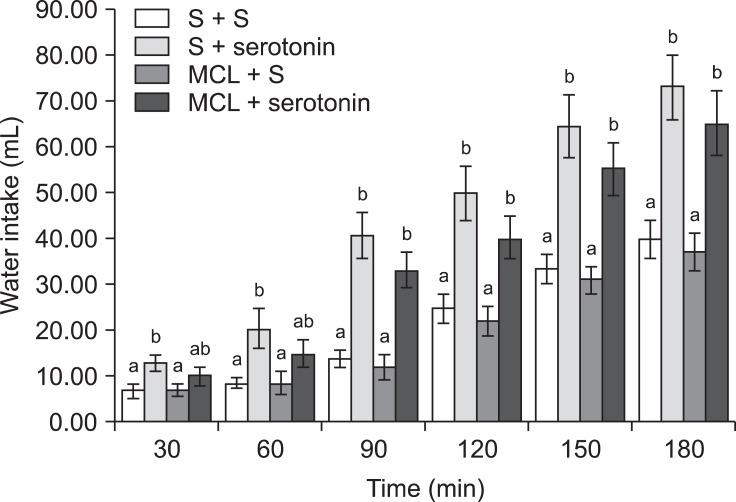
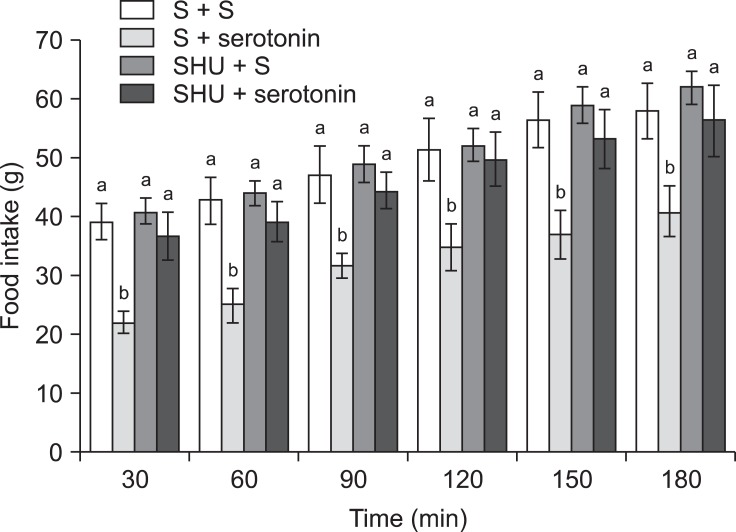
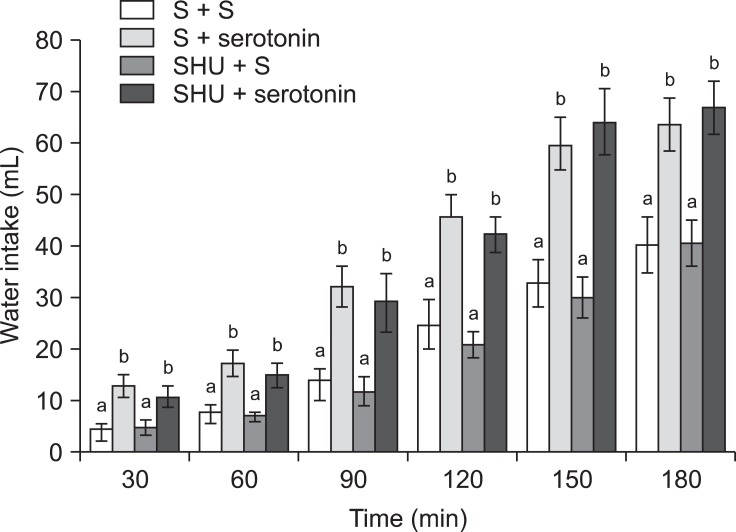
In Experiment 2, an ICV injection of 10 µg serotonin alone decreased food consumption but increased water intake (p ≤ 0.05) in FD24 chickens. On the other hand, 2 nmol SHU9119 alone had no effect on food or water intake (Fig. 3; p > 0.05). Furthermore, the effect of serotonin on food intake was significantly attenuated by pretreatment with 2 nmol SHU9119 [Fig. 3; f (3, 25) = 14.08; p ≤ 0.05]. However, SHU9119 did not alter the dipsogenic effect of serotonin (Fig. 5; p > 0.05).
Fig. 3.
Effect of ICV injection of SHU9119 (2 nmol) followed by serotonin (10 µg) on food intake in FD24 chickens. Data are presented as the mean ± SE. Lowercase letters (a and b) indicate significant differences between the treatments (p ≤ 0.05). S: saline, SHU: SHU9119.
Fig. 5.
Effects of ICV injection of SHU9119 (2 nmol) followed by serotonin (10 µg) on water intake in FD24 chickens. Data are presented as the mean ± SE. Lowercase letters (a and b) indicate significant differences between the treatments (p ≤ 0.05).
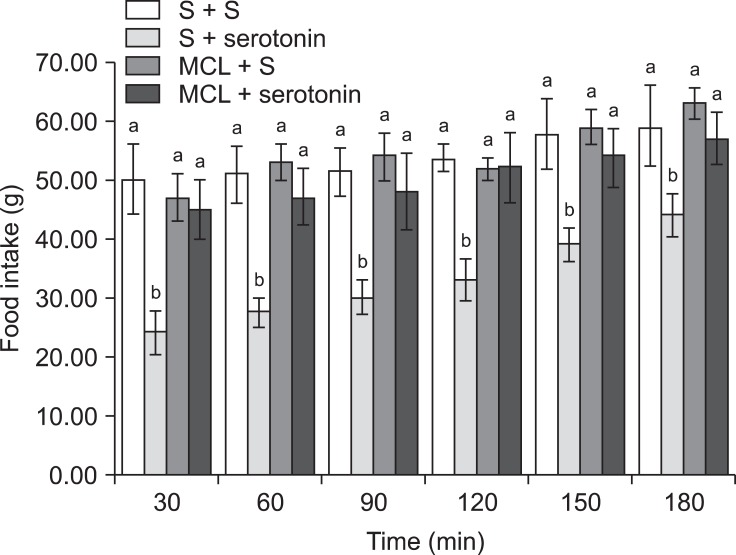
The results of Experiment 3 showed that the inhibitory effect of serotonin on cumulative food intake was significantly decreased by pretreatment with 2 nmol MCL0020 [Fig. 4; f (3, 25) = 18.56; p ≤ 0.05]. Additionally, MCL0020 had a modest effect on the dipsogenic response to serotonin [Fig. 6; f (3, 25) = 13.22; p ≤ 0.05]. The effect of MCL0020 alone on food and water intake was similar to that of SHU9119 (Fig. 6).
Fig. 4.
Effects of ICV injection of MCL0020 (2 nmol) followed by serotonin (10 µg) on food intake in FD24 chickens. Data are presented as the mean ± SE. Lowercase letters (a and b) indicate significant differences between the treatments (p ≤ 0.05). MCL: MCL0020.
Discussion
In chickens, serotonergic systems are involved in the regulation of numerous physiological functions. Many reports have indicated that serotonin circuitry affects feeding as well as drinking behaviors in pigeons, and that these changes are possibly mediated by independent mechanisms [2,4,15,23]. In the present study, serotonin decreased food intake but increases water consumption in FD24 broiler cockerels. Data from Experiment 1 indicated that serotonin circuitry is involved in the regulation of food as well as water intake in chickens, and suggested that these serotonergic effects are mediated by independent mechanisms. Therefore, species-associated differences in the avian serotonergic mechanism response may not only be restricted to feeding behavior, but also independently affect the mechanisms involved in drinking behavior.
The melanocortin system, particularly Mc4-R, modulates feeding behavior in mammals and birds. Heisler et al. [8] reported that the central melanocortin system in chickens appears to be important for regulating feeding activity because POMC mRNA expression is reduced with feed restriction. Mc3-R and Mc4-R both affect energy homeostasis through different mechanisms [9]. On one hand, Mc4-R knockout mice are hyperphagic and obese. In contrast, Mc3-R knockout mice develop a metabolic dysfunction characterized by elevated fat storage and decreased energy expenditure, but food intake and body weight remain unchanged [1]. These findings suggest that Mc3-R is important for body weight gain but the underlying mechanisms remain unclear [21]. In fact, ICV injection of the high-affinity Mc3-R agonist γ-MSH fails to inhibit food intake in rats, suggesting that the Mc3-R does not mediate changes in melanocortin-induced feeding behavior [10]. In addition, Takeuchi and Takahashi reported that Mc4-R is expressed in chicken brain but Mc3-R is not [26-28]. Concurrently, a selective Mc4-R antagonist, HS014, significantly increases food consumption in doves, indicating that Mc4-Rs mediate the orexigenic effects of endogenous agouti related peptide (AgRP).
In rats, HS014 is a potent inducer of feeding, and long-term administration of this compound results in obesity [10]. ICV injection of Mc3/4-R agonists in avian species (chickens and doves) has been shown to significantly decrease food intake [12]. Previous studies showed that the melanocortin agonist MTII suppresses the compensatory feeding responses of food-deprived doves but HS014 stimulates feeding activity when centrally administered [12,24,25]. However, Experiments 2 and 3 of the current study showed that treatment with melanocortin antagonists (SHU9119 and MCL0020) alone had no effect on feeding or drinking responses.
In 2000, Kask et al. [11] reported that α-MSH and β-MSH inhibit fasting-induced food intake by nearly 50% but neither of these two peptides inhibits fluid consumption in water-deprived (24 h) rats. Due to its possible role in controlling food intake, the central melanocortin system is an important downstream target for some hormones and signal transmitters such as insulin, leptin, ghrelin, and neuropeptide Y (NPY) that regulate feeding behavior in avian species [22]. Despite the established clinical effectiveness of serotonin-derived drugs for promoting weight loss, the mechanisms responsible for this effect in birds have remained obscure. Evidence from behavioral studies has shown that the central melanocortin system is the key site of serotonin action associated with food intake in chickens. serotonin has been shown to inhibit AgRP neuronal activity and reduce inhibitory postsynaptic currents onto POMC neurons via the serotonin1B receptor [7].
To determine whether melanocortin circuitry regulation is critical for serotonin-induced feeding and drinking responses, the impact of melanocortin (Mc3 and Mc4) antagonists on food and water consumption promoted by serotonin in FD24 broiler cockerels was examined. Results obtained from Experiments 2 and 3 showed that the anorexic effect of the serotonin ICV injection was attenuated by MCL0020 and SHU9119 pretreatment. Conversely, pretreatment with these two compounds had no effect on water intake in the chickens. These results showed that the effects of serotonin on food intake are modulated by the pathway(s) linked to the Mc4-Rs. Data from the current study indicated that both serotonin and Mc4-R affect the centers of that regulate feeding and energy balance.
Heisler et al. [8] previously showed that the melanocortin pathways are downstream targets for d-fenfluramine (an appetite suppressant that acts on serotonin2C receptor) and CP-94,253 (a high-affinity serotonin2C/1B receptor agonist) to promote satiety. A mechanism was subsequently proposed in which serotonin regulates feeding behavior via the melanocortin pathways. It has been suggested that some NPY/AgRP terminals are gamma aminobutyric acid and are in contact with nearby POMC neurons, thereby affecting POMC neuronal activity [22]. Clear support for a hypothetical brainstem locus for serotonin hypophagic action has been provided by researchers such as Lee et al. [17] and Grill et al. [6]. For example, injection of CP-93,129 into the lateral parabrachial nucleus (LPBN) reduces food intake in a dose-dependent manner. LPBN is a key site for autonomic regulation and Mc4-Rs are expressed in this region [13,18]. It is possible that serotonin may establish axo-axonal contacts with POMC and AgRP terminals arising from the arcuate nucleus that forms synaptic connections with cells in sites such as the LPBN that express Mc4-Rs [8]. Specifically, if serotonin affects POMC and AgRP neuronal activity, then the release of co-expressed neuropeptides such as cocaine and amphetamine-regulated transcript (CART) and NPY should also be affected. Both CART and NPY alter food intake and the release of these neuropeptides may enhance, interfere with, or have no effect on the actions of serotonin agonists [14,16].
In summary, the present study showed that pretreatment with MCL0020 and SHU9119 blocked serotonin hypophagia but did not alter serotonin induced hyperdipsia in FD24 chickens. These findings suggest that serotonin hypophagia and hyperdipsia were mediated by different mechanisms in the central nervous system, and that serotonin required downstream activation of McRs to promote hypophagia but not hyperdipsia in the FD24 chickens.
Acknowledgments
This research was supported by a grant from the Research Council of the Faculty of Veterinary Medicine, University of Tehran, Iran.
References
- 1.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van der Ploeg LHT. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 2.Denbow DM. Body temperature and food intake of turkeys following ICV injections of serotonin. Nutr Behav. 1983;1:301–308. [Google Scholar]
- 3.Denbow DM, Cherry JA, Siegel PB, Van Krey HP. Eating, drinking and temperature response of chicks to brain catecholamine injections. Physiol Behav. 1981;27:265–269. doi: 10.1016/0031-9384(81)90268-7. [DOI] [PubMed] [Google Scholar]
- 4.Denbow DM, Van Krey HP, Lacy MP, Dietrick TJ. Feeding, drinking and body temperature of Leghorn chicks: effects of ICV injections of biogenic amines. Physiol Behav. 1983;31:85–90. doi: 10.1016/0031-9384(83)90100-2. [DOI] [PubMed] [Google Scholar]
- 5.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 6.Grill HJ, Donahey JCK, King L, Kaplan JM. Contribution of caudal brainstem to d-fenfluramine anorexia. Psychopharmacology (Berl) 1997;130:375–381. doi: 10.1007/s002130050253. [DOI] [PubMed] [Google Scholar]
- 7.Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Marcus JN, Holstege H, Lee CE, Cone RD, Elmquist JK. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297:609–611. doi: 10.1126/science.1072327. [DOI] [PubMed] [Google Scholar]
- 8.Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thornton-Jones Z, Liu HY, Zigman JM, Balthasar N, Kishi T, Lee CE, Aschkenasi CJ, Zhang CY, Yu J, Boss O, Mountjoy KG, Clifton PG, Lowell BB, Friedman JM, Horvath T, Butler AA, Elmquist JK, Cowley MA. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 10.Kask A, Pähkla R, Irs A, Rägo L, Wikberg JES, Schiöth HB. Long-term administration of MC4 receptor antagonist HS014 causes hyperphagia and obesity in rats. Neuroreport. 1999;10:707–711. doi: 10.1097/00001756-199903170-00009. [DOI] [PubMed] [Google Scholar]
- 11.Kask A, Rägo L, Wikberg JES, Schiöth HB. Differential effects of melanocortin peptides on ingestive behaviour in rats: evidence against the involvement of MC3 receptor in the regulation of food intake. Neurosci Lett. 2000;283:1–4. doi: 10.1016/s0304-3940(00)00837-5. [DOI] [PubMed] [Google Scholar]
- 12.Kawakami S, Bungo T, Ando R, Ohgushi A, Shimojo M, Masuda Y, Furuse M. Central administration of α-melanocyte stimulating hormone inhibits fasting- and neuropeptide Y-induced feeding in neonatal chicks. Eur J Pharmacol. 2000;398:361–364. doi: 10.1016/s0014-2999(00)00344-7. [DOI] [PubMed] [Google Scholar]
- 13.Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- 14.Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, Larsen PJ, Hastrup S. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–76. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 15.Kuenzel WJ. Central neuroanatomical systems involved in the regulation of food intake in birds and mammals. J Nutr. 1994;124(8 Suppl):1355S–1370S. [PubMed] [Google Scholar]
- 16.Lambert PD, Couceyro PR, McGirr KM, Dall Vechia SE, Smith Y, Kuhar MJ. CART peptides in the central control of feeding and interactions with neuropeptide Y. Synapse. 1998;29:293–298. doi: 10.1002/(SICI)1098-2396(199808)29:4<293::AID-SYN1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Lee MD, Aloyo VJ, Fluharty SJ, Simansky KJ. Infusion of the serotonin1B (5-HT1B) agonist CP-93,129 into the parabrachial nucleus potently and selectively reduces food intake in rats. Psychopharmacology (Berl) 1998;136:304–307. doi: 10.1007/s002130050570. [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci. 2003;23:7143–7154. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludwig DS, Mountjoy KG, Tatro JB, Gillette JA, Frederich RC, Flier JS, Maratos-Flier E. Melanin-concentrating hormone: a functional melanocortin antagonist in the hypothalamus. Am J Physiol. 1998;274:E627–E633. doi: 10.1152/ajpendo.1998.274.4.E627. [DOI] [PubMed] [Google Scholar]
- 20.Olanrewaju HA, Thaxton JP, Dozier WA, 3rd, Purswell J, Roush WB, Branton SL. A review of lighting programs for broiler production. Int J Poult Sci. 2006;5:301–308. [Google Scholar]
- 21.Raffin-Sanson ML, Bertherat J. Mc3 and Mc4 receptors: complementary role in weight control. Eur J Endocrinol. 2001;144:207–208. doi: 10.1530/eje.0.1440207. [DOI] [PubMed] [Google Scholar]
- 22.Roseberry AG, Liu H, Jackson AC, Cai X, Friedman JM. Neuropeptide Y-mediated inhibition of proopiomelanocortin neurons in the arcuate nucleus shows enhanced desensitization in ob/ob mice. Neuron. 2004;41:711–722. doi: 10.1016/s0896-6273(04)00074-1. [DOI] [PubMed] [Google Scholar]
- 23.Steffens SM, Casas DC, Milanez BC, Freitas CG, Paschoalini MA, Marino-Neto J. Hypophagic and dipsogenic effects of central 5-HT injections in pigeons. Brain Res Bull. 1997;44:681–688. doi: 10.1016/s0361-9230(97)00199-8. [DOI] [PubMed] [Google Scholar]
- 24.Strader AD, Schiöth HB, Buntin JD. The role of the melanocortin system and the melanocortin-4 receptor in ring dove (Streptopelia risoria) feeding behavior. Brain Res. 2003;960:112–121. doi: 10.1016/s0006-8993(02)03799-x. [DOI] [PubMed] [Google Scholar]
- 25.Tachibana T, Sugahara K, Ohgushi A, Ando R, Kawakami S, Yoshimatsu T, Furuse M. Intracerebroventricular injection of agouti-related protein attenuates the anorexigenic effect of alpha-melanocyte stimulating hormone in neonatal chicks. Neurosci Lett. 2001;305:131–134. doi: 10.1016/s0304-3940(01)01827-4. [DOI] [PubMed] [Google Scholar]
- 26.Takeuchi S, Takahashi S. Melanocortin receptor genes in the chicken-tissue distributions. Gen Comp Endocrinol. 1998;112:220–231. doi: 10.1006/gcen.1998.7167. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi S, Takahashi S. A possible involvement of melanocortin 3 receptor in the regulation of adrenal gland function in the chicken. Biochim Biophys Acta. 1999;1448:512–518. doi: 10.1016/s0167-4889(98)00165-7. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi S, Teshigawara K, Takahashi S. Widespread expression of Agouti-related protein (AGRP) in the chicken: a possible involvement of AGRP in regulating peripheral melanocortin systems in the chicken. Biochim Biophys Acta. 2000;1496:261–269. doi: 10.1016/s0167-4889(00)00022-7. [DOI] [PubMed] [Google Scholar]
- 29.Thurmon JC, Tranquilli WJ, Benson GJ, editors. Lumb and Jones' Veterinary Anesthesia. 3rd ed. Baltimore: Williams and Wilkins; 1996. pp. 686–735. [Google Scholar]
- 30.Van Tienhoven A, Juhász LP. The chicken telencephalon, diencephalon and mesencephalon in sterotaxic coordinates. J Comp Neurol. 1962;118:185–197. doi: 10.1002/cne.901180205. [DOI] [PubMed] [Google Scholar]