Abstract
Two mitochondrial genotypes are shown to exist within one Holstein cow maternal lineage. They were detected by the appearance of an extra Hae III recognition site in one genotype. The nucleotide sequence of this region has been determined and the genotypes are distinguished by an adenine/guanine base transition which creates the new Hae III site. This point mutation occurs within an open reading frame at the third position of a glycine codon and therefore does not alter the amino acid sequence. The present pattern of genotypes within the lineage demands that multiple shifts between genotypes must have occurred within the past 20 years with the most rapid shift taking place in no more than 4 years and indicates that mitochondrial DNA polymorphism can occur between maternally related mammals. The process that gave rise to different genotypes in one lineage is clearly of fundamental importance in understanding intraspecific mitochondrial polymorphism and evolution in mammals. Several potential mechanisms for rapid mitochondrial DNA variation are discussed in light of these results.
Full text
PDF
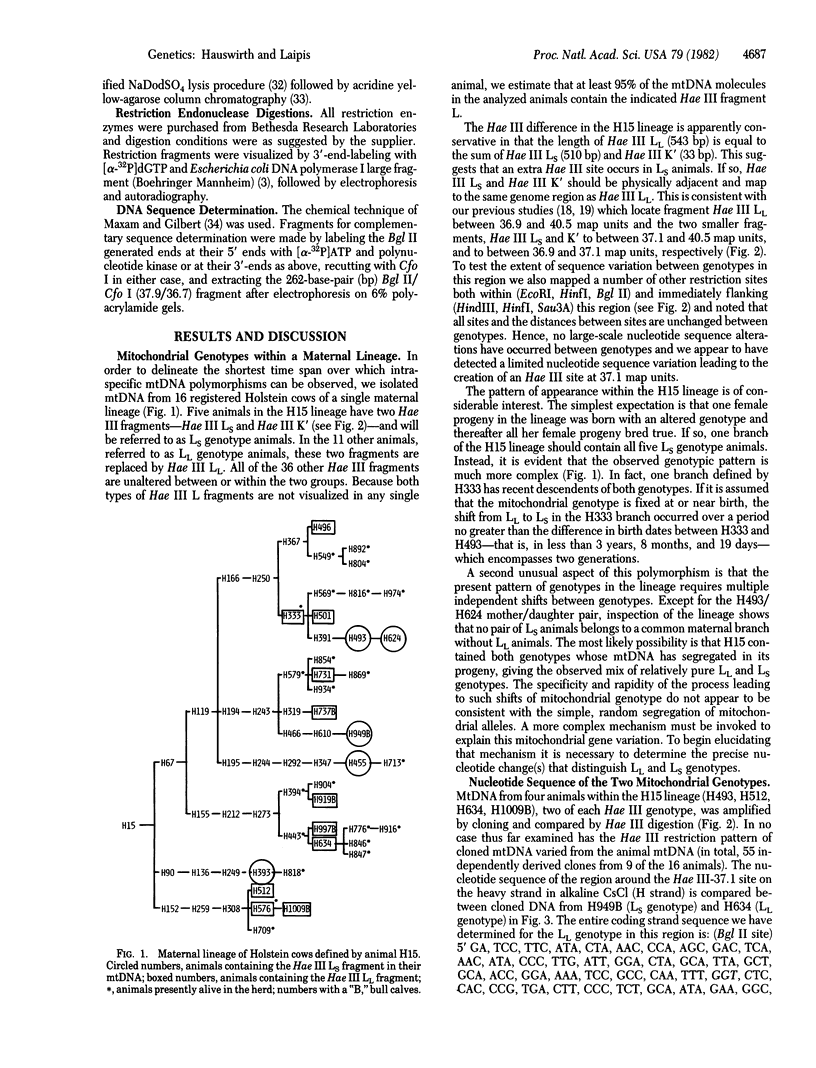
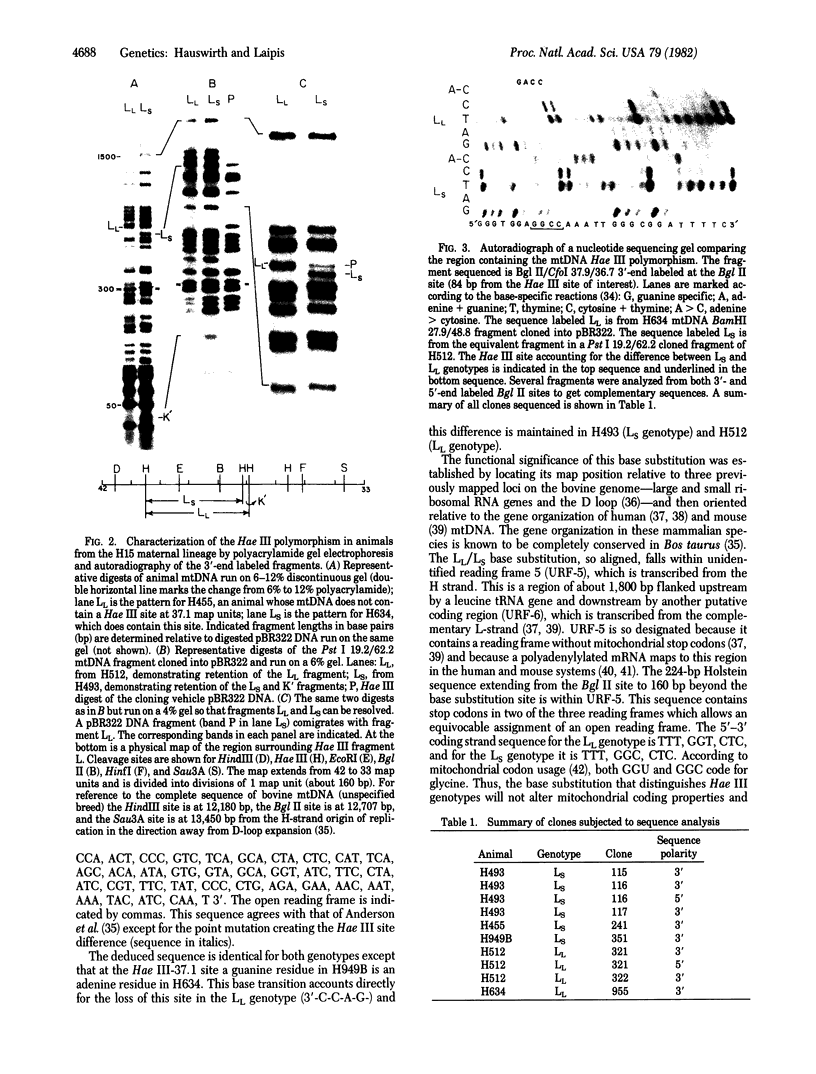


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J Mol Biol. 1982 Apr 25;156(4):683–717. doi: 10.1016/0022-2836(82)90137-1. [DOI] [PubMed] [Google Scholar]
- Barrell B. G., Anderson S., Bankier A. T., de Bruijn M. H., Chen E., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A. Different pattern of codon recognition by mammalian mitochondrial tRNAs. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3164–3166. doi: 10.1073/pnas.77.6.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battey J., Clayton D. A. The transcription map of mouse mitochondrial DNA. Cell. 1978 May;14(1):143–156. doi: 10.1016/0092-8674(78)90309-4. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Birky C. W., Jr Transmission genetics of mitochondria and chloroplasts. Annu Rev Genet. 1978;12:471–512. doi: 10.1146/annurev.ge.12.120178.002351. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc H., Wright C. T., Bibb M. J., Wallace D. C., Clayton D. A. Mitochondrial DNA of chloramphenicol-resistant mouse cells contains a single nucleotide change in the region encoding the 3' end of the large ribosomal RNA. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3789–3793. doi: 10.1073/pnas.78.6.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenhagen D., Clayton D. A. The number of mitochondrial deoxyribonucleic acid genomes in mouse L and human HeLa cells. Quantitative isolation of mitochondrial deoxyribonucleic acid. J Biol Chem. 1974 Dec 25;249(24):7991–7995. [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Brown G. G., Simpson M. V. Intra- and interspecific variation of the mitochondrial genome in Rattus norvegicus and Rattus rattus: restriction enzyme analysis of variant mitochondrial DNA molecules and their evolutionary relationships. Genetics. 1981 Jan;97(1):125–143. doi: 10.1093/genetics/97.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M., George M., Jr, Wilson A. C. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M. Polymorphism in mitochondrial DNA of humans as revealed by restriction endonuclease analysis. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3605–3609. doi: 10.1073/pnas.77.6.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzo K., Fouts D. L., Wolstenholme D. R. EcoRI cleavage site variants of mitochondrial DNA molecules from rats. Proc Natl Acad Sci U S A. 1978 Feb;75(2):909–913. doi: 10.1073/pnas.75.2.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bünemann H., Müller W. Base specific fractionation of double stranded DNA: affinity chromatography on a novel type of adsorbant. Nucleic Acids Res. 1978 Mar;5(3):1059–1074. doi: 10.1093/nar/5.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawid I. B., Blackler A. W. Maternal and cytoplasmic inheritance of mitochondrial DNA in Xenopus. Dev Biol. 1972 Oct;29(2):152–161. doi: 10.1016/0012-1606(72)90052-8. [DOI] [PubMed] [Google Scholar]
- Delbrück M., Ootaki T. An unstable nuclear gene in phycomyces. Genetics. 1979 May;92(1):27–48. doi: 10.1093/genetics/92.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris S. D., Sage R. D., Wilson A. C. Evidence from mtDNA sequences that common laboratory strains of inbred mice are descended from a single female. Nature. 1982 Jan 14;295(5845):163–165. doi: 10.1038/295163a0. [DOI] [PubMed] [Google Scholar]
- Francisco J. F., Brown G. G., Simpson M. V. Further studies on types A and B rat mtDNAs: cleavage maps and evidence for cytoplasmic inheritance in mammals. Plasmid. 1979 Jul;2(3):426–436. doi: 10.1016/0147-619x(79)90026-x. [DOI] [PubMed] [Google Scholar]
- Francisco J. F., Simpson M. V. The occurrence of two types of mitochondrial DNA in rat populations as detected by EcoRI endonuclease analysis. FEBS Lett. 1977 Jul 15;79(2):290–294. doi: 10.1016/0014-5793(77)80805-3. [DOI] [PubMed] [Google Scholar]
- Giles R. E., Blanc H., Cann H. M., Wallace D. C. Maternal inheritance of human mitochondrial DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6715–6719. doi: 10.1073/pnas.77.11.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J. M., Masters J. N., Jones S. S., Ashworth W. D., Jr, Wolstenholme D. R. Nucleotide sequence variants of Rattus norvegicus mitochondrial DNA. Chromosoma. 1981;82(5):595–609. doi: 10.1007/BF00285770. [DOI] [PubMed] [Google Scholar]
- Hauswirth W. W., Laipis P. J., Gilman M. E., O'Brien T. W., Michaels G. S., Rayfield M. A. Genetic mapping of bovine mitochondrial DNA from a single animal. Gene. 1980 Jan;8(2):193–209. doi: 10.1016/0378-1119(80)90037-2. [DOI] [PubMed] [Google Scholar]
- Hayashi J. I., Gotoh O., Tagashira Y. Length of polymorphisms of restriction fragments of rat mitochondrial DNAs. Biochem Biophys Res Commun. 1981 Feb 27;98(4):936–941. doi: 10.1016/0006-291x(81)91201-8. [DOI] [PubMed] [Google Scholar]
- Hayashi J. I., Yonekawa H., Gotoh O., Watanabe J., Tagashira Y. Strictly maternal inheritance of rat mitochondrial DNA. Biochem Biophys Res Commun. 1978 Aug 14;83(3):1032–1038. doi: 10.1016/0006-291x(78)91499-7. [DOI] [PubMed] [Google Scholar]
- Hayashi J., Yonekawa H., Gotoh O., Motohashi J., Tagashira Y. Two different molecular types of rat mitochondrial DNAs. Biochem Biophys Res Commun. 1978 Apr 14;81(3):871–877. doi: 10.1016/0006-291x(78)91432-8. [DOI] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Newbold J. E., Potter S. S., Edgell M. H. Maternal inheritance of mammalian mitochondrial DNA. Nature. 1974 Oct 11;251(5475):536–538. doi: 10.1038/251536a0. [DOI] [PubMed] [Google Scholar]
- Kearsey S. E., Craig I. W. Altered ribosomal RNA genes in mitochondria from mammalian cells with chloramphenicol resistance. Nature. 1981 Apr 16;290(5807):607–608. doi: 10.1038/290607a0. [DOI] [PubMed] [Google Scholar]
- King B. O., Shade R. O., Lansman R. A. The use of restriction endonucleases to compare mitochondrial DNA sequences in Mus musculus: a detailed restriction map of mitochondrial DNA from mouse L cells. Plasmid. 1981 May;5(3):313–328. doi: 10.1016/0147-619x(81)90008-1. [DOI] [PubMed] [Google Scholar]
- Kroon A. M., Pepe G., Bakker H., Holtrop M., Bollen J. E., Van Bruggen E. F., Cantatore P., Terpstra P., Saccone C. The restriction fragment map of rat-liver mitochondrial DNA: a reconsideration. Biochim Biophys Acta. 1977 Sep 20;478(2):128–145. doi: 10.1016/0005-2787(77)90177-0. [DOI] [PubMed] [Google Scholar]
- Kroon A. M., de Vos W. M., Bakker H. The heterogeneity of rat-liver mitochondrial DNA. Biochim Biophys Acta. 1978 Jun 22;519(1):269–273. doi: 10.1016/0005-2787(78)90079-5. [DOI] [PubMed] [Google Scholar]
- Laipis P. J., Hauswirth W. W., O'Brien T. W., Michaels G. S. A physical map of bovine mitochondrial DNA from a single animal. Biochim Biophys Acta. 1979 Nov 22;565(1):22–32. doi: 10.1016/0005-2787(79)90080-7. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nass M. M. Restriction endonuclease analysis of mitochondrial DNA from virus-transformed, tumor and control cells of human, hamster and avian origin. Sequence conservation and intraspecific variation. Biochim Biophys Acta. 1981 Sep 28;655(2):210–220. doi: 10.1016/0005-2787(81)90011-3. [DOI] [PubMed] [Google Scholar]
- Ojala D., Merkel C., Gelfand R., Attardi G. The tRNA genes punctuate the reading of genetic information in human mitochondrial DNA. Cell. 1980 Nov;22(2 Pt 2):393–403. doi: 10.1016/0092-8674(80)90350-5. [DOI] [PubMed] [Google Scholar]
- Ojala D., Montoya J., Attardi G. tRNA punctuation model of RNA processing in human mitochondria. Nature. 1981 Apr 9;290(5806):470–474. doi: 10.1038/290470a0. [DOI] [PubMed] [Google Scholar]
- Pikó L., Matsumoto L. Number of mitochondria and some properties of mitochondrial DNA in the mouse egg. Dev Biol. 1976 Mar;49(1):1–10. doi: 10.1016/0012-1606(76)90253-0. [DOI] [PubMed] [Google Scholar]
- Potter S. S., Newbold J. E., Hutchison C. A., 3rd, Edgell M. H. Specific cleavage analysis of mammalian mitochondrial DNA. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4496–4500. doi: 10.1073/pnas.72.11.4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upholt W. B., Dawid I. B. Mapping of mitochondrial DNA of individual sheep and goats: rapid evolution in the D loop region. Cell. 1977 Jul;11(3):571–583. doi: 10.1016/0092-8674(77)90075-7. [DOI] [PubMed] [Google Scholar]
- de Vos W. M., Bakker H., Saccone C., Kroon A. M. Further analysis of the type differences of rat-liver mitochondrial DNA. Biochim Biophys Acta. 1980 Mar 28;607(1):1–9. doi: 10.1016/0005-2787(80)90215-4. [DOI] [PubMed] [Google Scholar]