Abstract
Although adoptive T cell therapy (ACT) has become a promising immunotherapeutic regime for cancer treatment, its effectiveness has been hindered by several inherent shortcomings regarding safety and efficacy. During the past few decades, several strategies for enhancing the efficacy of ACT have been developed and introduced in clinic. This review will summarize not only the past approaches but also the latest strategies which have been shown to enhance the anticancer activity of ACT.
Keywords: Adoptive T cell therapy, Gene modification, Immunotherapy, Cancer
INTRODUCTION
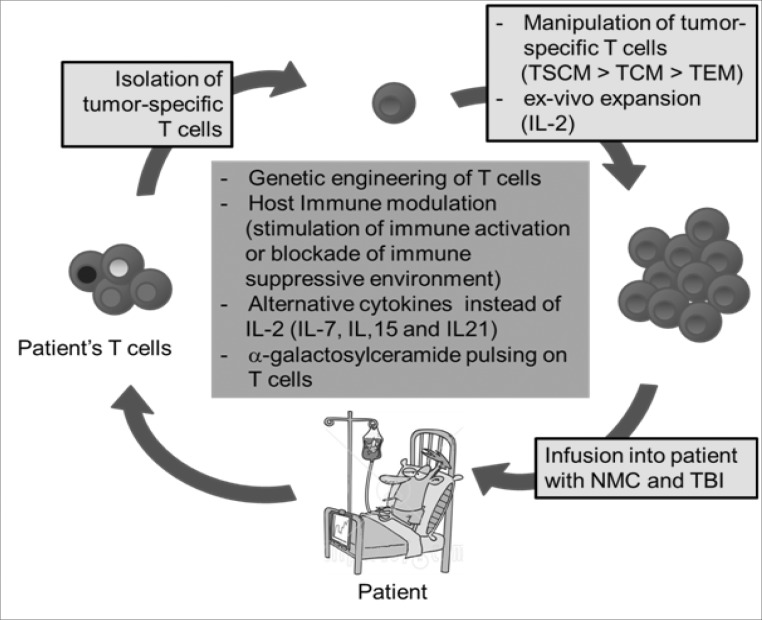
ACT is based on the transfer of ex-vivo expanded anti-tumor or anti-viral CD8 T cells into affected patients and has shown positive results in clinical trials (Fig. 1) (1). Historically, the concept of ACT was first introduced when a paper in 1964 demonstrated that the transfer of immune lymphocytes could inhibit growth of a carcinogen-induced rat sarcoma (2). The effectiveness of ACT was later enhanced by use of recombinant IL-2; it was the first breakthrough in ACT due to IL-2's ability to induce T cell stimulation and proliferation. Although recombinant IL-2 treatment need to be used carefully in clinic by toxicity such as 'capillary permeability leak syndrome' that results in major fluid retention, it had been previously approved by the Food and Drug Administration (FDA) for the treatment of metastatic renal cancer in 1992 and metastatic melanoma patients in 1998 (1).
Figure 1.
Schematic view of Adoptive T cell therapy. TSCM, stem cell-like memory T cells; TCM, central memory T cells; TEM, effector memory T cells; NMC, non-myeloablative chemotherapy; TBI, total body irradiation.
IL-2 characteristically enhances immunological functions. In the context of cancer, IL-2 can be used as a T cell growth factor in in vitro culture, and the intravenous injection of tumor-sensitized lymphocytes grown in long term culture in the presence of IL-2 is capable of curing mice with established local and disseminated syngeneic tumor (3). Moreover, systemic administration of IL-2 with tumor-sensitized lymphocytes exhibited enhanced therapeutic potential in an established tumor model of mice (4). Later, lymphocytes grown in culture with IL-2 were referred to as lymphokine-activated killer (LAK) cells. However, administration of a high dose of IL-2 and LAK cells was not shown to be superior in survival to that of IL-2 alone when patients had metastatic cancer (5).
In 1986, Rosenberg et al. showed that an adoptive transfer of tumor-infiltrating lymphocytes (TIL) was 50 to 100 times more effective than that of LAK cells (6). Moreover, IL-2 administration in combination with TIL could overcome limitations attributed to the use of TIL only, such as lowered activation or suppression of TIL. For this reason, in most current protocols, a high-dose of IL-2 is administered intravenously. Subsequently, the first clinical trial of TIL infusion with IL-2 administration for metastatic melanoma patients was published in 1988 (7). Despite promising initial results, treatment with TILs and IL-2 resulted in objective responses in only about one third of patients with metastatic melanoma (8). Currently, the expanded TIL cultures were selected by tumor-antigens for highly active tumor-specific T cells to enhance the efficacy of ACT with TIL (9,10).
The induction of immuno-depleting condition in patients before ACT by using non-myeloablative chemotherapy (NMC) and total body irradiation (TBI) was the second breakthrough in the field of ACT (1). Previous studies have already demonstrated a correlation between immune-depleting conditions and the efficacy of ACT (11-15). For example, immuno-depleting conditions by NMC using cyclophosphamide and fludarabine provided an optimal environment for infused TILs by eliminating suppressive regulatory T cells (Treg) and reducing competition with endogenous lymphocytes to receive the signal of homeostatic cytokines such as IL-7 and IL-15. Additionally, TBI can further augment lympho-depleting condition. Using ACT in the setting of NMC was demonstrated to mediate the regression of established tumors in ~50% of malignant melanoma patients (16).
The third breakthrough in ACT occurred when the differentiation properties of T cells were defined. Effector T cells differentiating in the later stages of long-term culture were found to be less effective in in vivo tumor treatment (17). This finding challenged the methodology of using long-term culture of T cells. However, Berger et al. demonstrated that CD8 T cells derived from central memory T cells (TCM), but not effector memory T cells (TEM), persisted long-term in vivo in macaques and reacquired phenotypic and functional properties of memory T cells and occupied memory T cell niches (18). Thus, TCMs were more effective than TEMs in enhancing ACT efficacy. More recently, Gattinoni et al. also identified stem cell-like memory T cells (TSCM) in humans, which consistently express a surface marker typically found on naïve T cells. However, unlike naïve T cells, TSCMs express Sca-1, Bcl-2, IL2Rβ and CXCR3 (19). TSCMs were shown to display greater antitumor effect than TCM when adoptively transferred into tumor bearing mice, which indicates TSCMs may play a greater role in future ACTs (19,20).
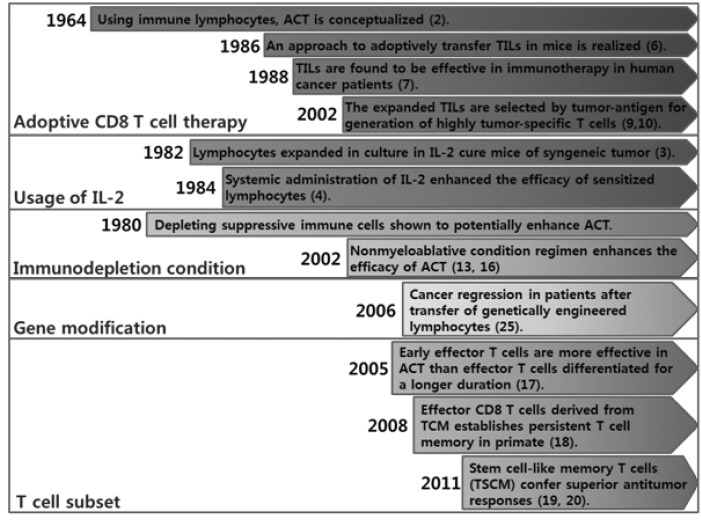
These three breakthroughs appeared to significantly improve ACT efficacy (Fig. 2). However, the ACT approach is not still satisfactory and needs to be further developed to effectively treat hard-to-cure cancer patients in clinics. The next sections will enumerate novel strategies that may make the ACT approach successful for clinical purposes in the near future.
Figure 2.
Timeline of adoptive CD8 T cell therapy development.
GENETIC MODIFICATION OF T CELLS
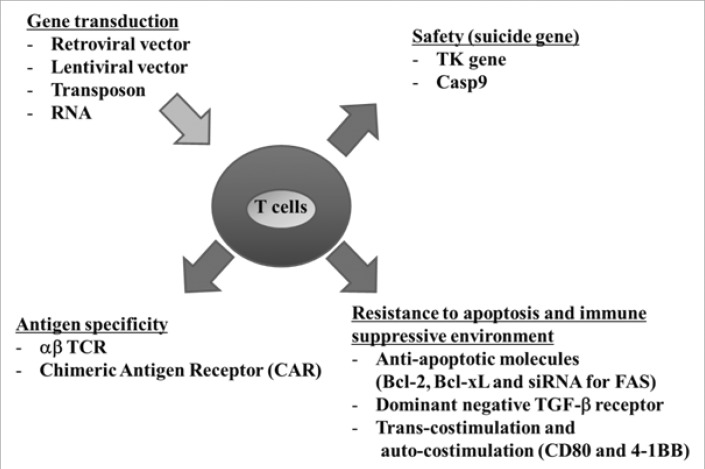
Recently, gene modification of T cells has also been utilized for enhancing the efficacy of ACT (Fig. 2). The transduction of α and β TCR was first introduced to confer reactivity against tumor-associated antigen (TAA). Genes encoding TCRs can be isolated from T cells with a high avidity for recognizing TAA. And, the retroviral or lentiviral vectors can be used to deliver TCR genes and redirect lymphocyte specificity to TAA (Fig. 3). This procedure allows the rapid production of TAA-specific T cells and has been applied to several antigens such as minor histocompatibility antigen (21), CEA (22), gp100 (23,24), MART-1 (25), p53 (26), NY-ESO-1 (27,28), WT-1 (29) and AURKA (30). The aforementioned approach was reported to be put into clinical trial in 2006 for the first time (25). In this trial, Morgan et al. reported that this method was indeed feasible by showing durable engraftment at levels exceeding 10% of peripheral blood lymphocytes of adoptively transferred genetically modified MART-1-specific T cells into 15 patients. In two of the patients, the engineered cells were maintained in the blood even 1 year after infusion and continued to demonstrate objective regression of metastatic melanoma lesions.
Figure 3.
Schematic view of gene modification of infused T cells.
Despite a positive outlook of α and β TCR transduction, Johnson et al. reported the possibility of toxicity by adoptive transfer of engineered TAA-specific T cells (31). When engineered TAA-specific T cells were administered to 36 patients with metastatic melanoma, engineered cells persisted at high levels in the blood of all patients at 1 month after infusion as expected and approximately 20 to 30% of patients exhibited objective cancer regression. However, though these initial results were promising, patients also exhibited a destruction of normal melanocytes in the skin, eye, and ear and sometimes required local steroid administration to treat uveitis and hearing loss. In addition, Parkhurst et al. recently reported in 2011 that engineered T cells mediate regression of metastatic colorectal cancer but induce severe transient colitis in patients (32). Thus, highly reactive TAA-specific T cells can mediate cancer-regression, but also target rare cognate-antigen-containing normal tissue throughout the body.
Although there is no clinical report, the cross pairing of transduced TCR chains and endogenous TCR chains, which form hybrid TCRs, may mediate unexpected autoimmune reactivity (33). Thus, several reports have already suggested strategies to prevent cross pairing of transduced TCR chains and endogenous TCR chains. Firstly, hybrid TCRs, which consists of a human variable region and murine constant region, can prevent cross pairing and are overexpressed on the surface when transduced into human lymphocytes (34,35). Preferential pairing of murine constant regions and improved CD3 stability seemed to prevent cross pairing. Secondly, the introduction of an additional disulfide bond into the α-and β-chains of TCRs improves preferential pairing with each other, increases total surface expression of transduced TCR chains, and reduces cross pairing with endogenous TCR chains (29,36,37). Thirdly, mutations in the transduced TCR structure promotes selective assembly of transduced TCRs while preserving its specificity and avidity for Ag ligands (38). Fourthly, the incorporation of CD3ζ into transduced TCRs results in highly preferred pairing between TCRα:CD3ζ and TCRs:CD3ζ and also prevents TCR cross pairing with TCR-chains: CD3ζand endogenous TCR chains (39,40). Fifthly, using a vector system encoding siRNAs for endogenous TCR genes can prevent the expression of endogenous TCRs (41). In this case, it is possible to prevent the reactivity of engineered T cells to normal cells by endogenous self-recognized TCR. Finally, transduction of engineered TCR genes into γδ T cells can prevent cross-pairing with transduced TCRs and endogenous TCRs in addition to maintaining their functionality, which includes specific proliferation capacity, Ag specific reactivity, in vivo persistence, and recall response (42).
However, the use of artificial αβ TCRs has a fundamental limitation because some tumors display lower levels of MHC class I molecules, resulting in poor efficacy of ACT by engineered T cells. Thus, the chimeric antigen receptor (CAR) was introduced to compensate for this limitation. CAR is generated by joining the light and heavy chain variable regions of a monoclonal antibody, referred to as a single chain Fc (scFv) molecule, with the hinge domain, transmembrane, and cytoplasmic signaling domains derived from the CD3ζ chain or Fc receptorγ chains. CAR directly recognizes cell-surface antigens and confers specificity of engineered T cells independently of antigen processing or MHC-restricted presentation. An example of a CAR target antigen was well summarized in a review by Sadelain (43). The first generation of CARs, whose signaling domain contained CD3ζ or FcRγ, has been shown to deliver a potent T cell activation signal. However, it was not sufficient enough in subsequent activation steps in the absence of a concomitant costimulatory signal (44). As a result, the second generation of CARs contained the signal transduction domain of CD28, 4-1BB, or other costimulatory signaling domains, which enhanced the therapeutic potential of engineered T cells (44-47). The most recent third generation of CARs contains triple-fusion signaling domains to enhance the therapeutic potential of engineered T cells (48).
An additional strategy to enhance the efficacy of ACT is transduction of anti-apoptotic molecules (Fig. 3). An intrinsic death pathway can be blocked by overexpression of Bcl-2. As evidence, tumor-specific T cells overexpressing Bcl-2 by gene transduction maintained their potential to recognize their target and were resistant to apoptosis (49). Moreover, anti-apoptotic molecule, Bcl-xL, is not expressed in resting T cells, but CD28 co-stimulation can induce transient expression of Bcl-xL, leading to resistance of Fas-induced apoptosis. Therefore, Bcl-xL transduction into T cells will also result in enhanced persistence of engineered T cells in vivo with thereby potentially improving the efficacy of ACT (50). Transduction of siRNA for FAS also makes engineered T cells resist FAS-induced apoptosis (Fig. 3) (51). Secondly, CD8 T cells did not become senescent when they were transduced with human telomerase (hTERT) (52), which is responsible for ensuring that the length of telomeres is relatively consistent, and led to persistence of engineered T cells in vivo (53). Thirdly, when dominant negative TGF-βRII was transduced into T cells, the engineered T cells resisted the inhibitory effects of tumor-derived TGF-β (Fig. 3) (54). TGF-β is an immunosuppressive cytokine produced in most human tumors and is known to markedly inhibit tumor-specific T cell responses, which is one of several immune response evasion strategies of tumors. Lastly, T cells transduced with some selected costimulatory molecules, CD80 and 4-1BBL, showed the potent tumor elimination of large and systemic tumors in immunodeficient mice (55). The engineered T cells can provide agonistic costimulatory signals to tumor-infiltrating T cells (trans-costimulation) and also provoke auto-costimulation to overcome adverse tumor microenvironment.
SAFETY ISSUES OF GENETICALLY-ENGINEERED T CELLS
In 2010, one case report suggested that autologous HER2-specific CAR-engineered T cells were also detrimentally toxic (56). In the case report, following completion of NMC, a patient received 1010 HER2-specific CAR-engineered T cells intravenously. Within 15 minutes after the cell infusion the patient experienced respiratory distress and displayed dramatic pulmonary infiltrate in chest X-rays. Despite intensive medical intervention the patient died 5 days later. Serum samples of the patient after infusion showed marked increases in IFN-γ, GM-CSF, TNF-α, IL-6 and IL-10 implying that over-reactivity of HER2-specific CAR-engineered T cells on a small amount of HER2 expressed in normal tissue. This case report demonstrated that safety should be of great concern in preventing graft-versus-host disease (GVHD) when using engineered T cells.
As a consequence, transduction of suicide genes, such as the herpes simplex virus thymidine kinase (HSV-tk), is suggested to control potential GVHD cases as a safety precaution (Fig. 3) (57). Using the HSV-tk suicide gene in engineered T cells, for instance, allows for administration of GCV when GVHD occurs; GCV then induces the apoptosis of the HSV-tk transduced T cells. However, one disadvantage of HSV-tk is that it is foreign by nature, and immune responses to HSV-tk transduced T cells could lead to their premature elimination after infusion (58,59). This is supported by evidence showing that, after infusion of HSV-tk transduced engineered T cells, HSV-tk-specific T cells were detected in the blood; repeated infusion of HSV-tk transduced engineered T cells induced memory HSV-tk-specific T cells (60). Thus, when HSV-tk transduced engineered T cells persist in vivo, they are a small fraction and contain HSV-tk transgene deletion by an anti-HSV-tk immune response after infusion (61).
Other than HSV-tk transduction, there are other, alternative strategies to prevent GVHD; firstly, human thymidylate kinase (62), human caspase 9 (63), or human FAS gene (64) can be transduced into autologous T cells to induce apoptosis of transduced T cells when GVHD occurs. Secondly, Griffioen et al. has shown that transduction of CD20 into T cells as a suicide mechanism efficiently induced elimination of engineered T cells by complement dependent cytotoxicity after treatment of therapeutic anti-CD20 antibody named rituximab (65). Thirdly, to circumvent these removal strategies, the use of RNA transfer technology is suggested for the benefit of safety concern. This is supported with the finding that when RNA is transferred into primary T cells, the expression of targeted protein is transient and gradually decreasing. Using RNA encoding CIR(chimeric immune receptor) against Her-2/neu antigens, antitumor effect was showed compared with Herceptin in vivo (66).
Additional strategy for enhancing the efficacy of ACT
Several strategies were introduced to enhance the efficacy of ACT; the first method is inhibition of the immunosuppressive environment in vivo. Selective depletion of regulatory T cells and blockade antibodies of cell surface inhibitory molecules (CTLA-4/PD-1) or inhibitory cytokine TGF-β secreted from tumor cells can enhance the efficacy of infused T cells (67). The second method is to enhance the functionality of infused T cells indirectly. Additional vaccination with peptide/virus/APC containing antigens after infusion of T cells can stimulate the infused T cells (68-70). Moreover, administration of TLR agonists further enhance the efficacy of infused T cells, followed by a long-term cure of large tumors (71). In addition, alternative cytokines, such as IL-7, IL-15 and IL-21 have been tested to increase the efficacy of infused T cells instead of IL-2 (72).
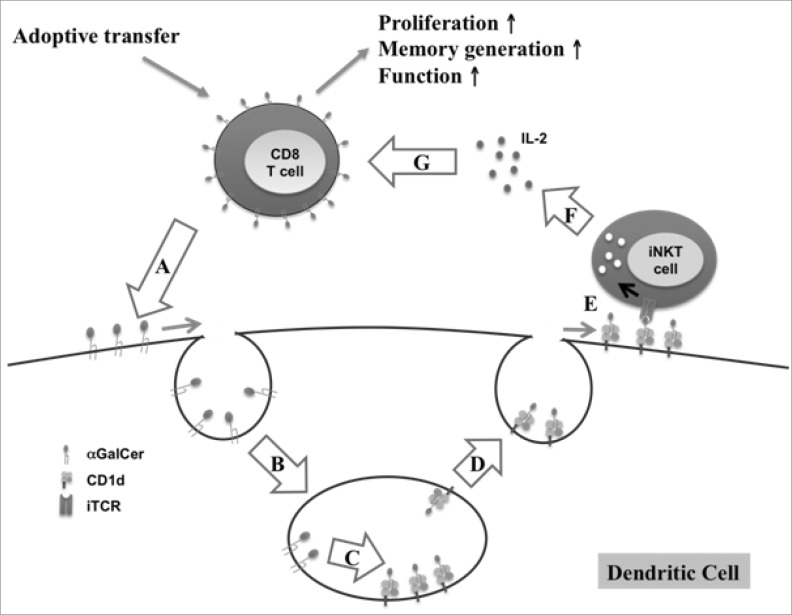
Recently, α-Galactosylceramide (αGalCer) was introduced to enhance the efficacy of ACT. When activated T cells were pulsed with αGalCer before infusion, the infused activated CD8 T cells markedly proliferated, secreted more cytokines, exhibited cytotoxic lymphocyte activity, and persisted for longer durations (73). Because αGalCer is known as a NKT cell ligand presented on CD1d (MHC class I like molecule), it induces NKT cells to secrete both Th1 and Th2 cytokines. These secreted cytokines activate a variety of other cell types, including NK cells, DCs, B cells and T cells (74). Fig. 4 suggests a possible action mechanism for αGalCer-pulsed CD8 T cells in ACT. First, αGalCer incorporated into the membrane of infused CD8 T cells is transferred into the membrane of DCs in a cell-to-cell contact manner. Subsequently, the transferred αGalCer is internalized via endocytosis into the endosomes of DCs. Thereafter, αGalCer associates with CD1d of DCs, perhaps through assistance by lipid transfer protein (LTP) (75,76). Afterwards, αGalCer-CD1d complexes are transported from the endosome to the cell surface for presentation to iNKT cells (77) where activated iNKT cells by αGalCer on DCs secrete IL-2, which enhance the proliferation of infused CD8 T cells.
Figure 4.
Action mechanism for enhanced efficacy of infused CD8 T cells by αGalCer-loading. (A) αGalCer transfer from activated CD8 T cells to DCs via cell-to-cell contact. (B) Internalization of αGalCer via endocytosis into endosome. (C) αGalCer loading on CD1d by LTP. (D) Transport of αGalCer-CD1d complexes from endosome to DC surface. (E) Presentation of αGalCer to iNKT cells. (F) Secretion of IL-2 by iNKT cells. (G) Enhanced proliferation and functionality of CD8 T cells by IL-2.
CONCLUSION
Currently, ACT is one of the most effective cell therapies with objective responses in more than 50% of the cancer patients when used in parallel with IL-2 and immunodepleting regimens. Moreover, the introduction of gene modification of T cells further enhanced ACT efficacy though there remain safety concerns using this methodology. Recently, using stimulatory glycolipid pulsing on infused T cells and combination with host immune modulators have been demonstrated to significantly increase the efficacy of ACT. Therefore, the combinatory effects of several therapeutic methods including genetic modification, αGalCer-pulsing, and other immunotherapies will be used as a practical approach to treat effectively hard-to-cure cancer patient in the near future.
ACKNOWLEDGEMENTS
This research was supported by the World Class University (WCU) program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (R31-2008-000-10105-0 or R31-10105).
We thank Han Park for helping with English editing.
Footnotes
The authors have no financial conflict of interest.
References
- 1.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delorme EJ, Alexander P. Treatment of primary fibrosarcoma in the rat with immune lymphocytes. Lancet. 1964;2:117–120. doi: 10.1016/s0140-6736(64)90126-6. [DOI] [PubMed] [Google Scholar]
- 3.Eberlein TJ, Rosenstein M, Rosenberg SA. Regression of a disseminated syngeneic solid tumor by systemic transfer of lymphoid cells expanded in interleukin 2. J Exp Med. 1982;156:385–397. doi: 10.1084/jem.156.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donohue JH, Rosenstein M, Chang AE, Lotze MT, Robb RJ, Rosenberg SA. The systemic administration of purified interleukin 2 enhances the ability of sensitized murine lymphocytes to cure a disseminated syngeneic lymphoma. J Immunol. 1984;132:2123–2128. [PubMed] [Google Scholar]
- 5.Rosenberg SA, Lotze MT, Yang JC, Topalian SL, Chang AE, Schwartzentruber DJ, Aebersold P, Leitman S, Linehan WM, Seipp CA, et al. Prospective randomized trial of high-dose interleukin-2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer. J Natl Cancer Inst. 1993;85:622–632. doi: 10.1093/jnci/85.8.622. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86:1159–1166. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 9.Seiter S, Monsurro V, Nielsen MB, Wang E, Provenzano M, Wunderlich JR, Rosenberg SA, Marincola FM. Frequency of MART-1/MelanA and gp100/PMel17-specific T cells in tumor metastases and cultured tumor-infiltrating lymphocytes. J Immunother. 2002;25:252–263. doi: 10.1097/01.CJI.0000015209.05216.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26:332–342. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muranski P, Boni A, Wrzesinski C, Citrin DE, Rosenberg SA, Childs R, Restifo NP. Increased intensity lymphodepletion and adoptive immunotherapy--how far can we go? Nat Clin Pract Oncol. 2006;3:668–681. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, Rosenberg SA, Restifo NP. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, Theofilopoulos AN. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110:185–192. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, Rosenberg SA, Restifo NP. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wrzesinski C, Paulos CM, Gattinoni L, Palmer DC, Kaiser A, Yu Z, Rosenberg SA, Restifo NP. Hematopoietic stem cells promote the expansion and function of adoptively transferred antitumor CD8 T cells. J Clin Invest. 2007;117:492–501. doi: 10.1172/JCI30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klebanoff CA, Gattinoni L, Palmer DC, Muranski P, Ji Y, Hinrichs CS, Borman ZA, Kerkar SP, Scott CD, Finkelstein SE, Rosenberg SA, Restifo NP. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin Cancer Res. 2011;17:5343–5352. doi: 10.1158/1078-0432.CCR-11-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bleakley M, Riddell SR. Exploiting T cells specific for human minor histocompatibility antigens for therapy of leukemia. Immunol Cell Biol. 2011;89:396–407. doi: 10.1038/icb.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parkhurst MR, Joo J, Riley JP, Yu Z, Li Y, Robbins PF, Rosenberg SA. Characterization of genetically modified T-cell receptors that recognize the CEA:691-699 peptide in the context of HLA-A2.1 onhuman colorectal cancer cells. Clin Cancer Res. 2009;15:169–180. doi: 10.1158/1078-0432.CCR-08-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan RA, Dudley ME, Yu YY, Zheng Z, Robbins PF, Theoret MR, Wunderlich JR, Hughes MS, Restifo NP, Rosenberg SA. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J Immunol. 2003;171:3287–3295. doi: 10.4049/jimmunol.171.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaft N, Willemsen RA, de Vries J, Lankiewicz B, Essers BW, Gratama JW, Figdor CG, Bolhuis RL, Debets R, Adema GJ. Peptide fine specificity of anti-glycoprotein 100 CTL is preserved following transfer of engineered TCR alpha beta genes into primary human T lymphocytes. J Immunol. 2003;170:2186–2194. doi: 10.4049/jimmunol.170.4.2186. [DOI] [PubMed] [Google Scholar]
- 25.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theoret MR, Cohen CJ, Nahvi AV, Ngo LT, Suri KB, Powell DJ, Jr, Dudley ME, Morgan RA, Rosenberg SA. Relationship of p53 overexpression on cancers and recognition by anti-p53 T cell receptor-transduced T cells. Hum Gene Ther. 2008;19:1219–1232. doi: 10.1089/hum.2008.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krönig H, Hofer K, Conrad H, Guilaume P, Müller J, Schiemann M, Lennerz V, Cosma A, Peschel C, Busch DH, Romero P, Bernhard H. Allorestricted T lymphocytes with a high avidity T-cell receptor towards NY-ESO-1 have potent anti-tumor activity. Int J Cancer. 2009;125:649–655. doi: 10.1002/ijc.24414. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Y, Zheng Z, Robbins PF, Khong HT, Rosenberg SA, Morgan RA. Primary human lymphocytes transduced with NY-ESO-1 antigen-specific TCR genes recognize and kill diverse human tumor cell lines. J Immunol. 2005;174:4415–4423. doi: 10.4049/jimmunol.174.7.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas S, Xue SA, Cesco-Gaspere M, San Jose E, Hart DP, Wong V, Debets R, Alarcon B, Morris E, Stauss HJ. Targeting the Wilms tumor antigen 1 by TCR gene transfer: TCR variants improve tetramer binding but not the function of gene modified human T cells. J Immunol. 2007;179:5803–5810. doi: 10.4049/jimmunol.179.9.5803. [DOI] [PubMed] [Google Scholar]
- 30.Nagai K, Ochi T, Fujiwara H, An J, Shirakata T, Mineno J, Kuzushima K, Shiku H, Melenhorst JJ, Gostick E, Price DA, Ishii E, Yasukawa M. Aurora kinase A-specific T-cell receptor gene transfer redirects T lymphocytes to display effective antileukemia reactivity. Blood. 2011;119:368–376. doi: 10.1182/blood-2011-06-360354. [DOI] [PubMed] [Google Scholar]
- 31.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich JR, Lee CC, Restifo NP, Schwarz SL, Cogdill AP, Bishop RJ, Kim H, Brewer CC, Rudy SF, VanWaes C, Davis JL, Mathur A, Ripley RT, Nathan DA, Laurencot CM, Rosenberg SA. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM, Hughes MS, Kammula US, Phan GQ, Lim RM, Wank SA, Restifo NP, Robbins PF, Laurencot CM, Rosenberg SA. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19:620–626. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bendle GM, Linnemann C, Hooijkaas AI, Bies L, de Witte MA, Jorritsma A, Kaiser AD, Pouw N, Debets R, Kieback E, Uckert W, Song JY, Haanen JB, Schumacher TN. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med. 2010;16:565–570. doi: 10.1038/nm.2128. [DOI] [PubMed] [Google Scholar]
- 34.Cohen CJ, Zhao Y, Zheng Z, Rosenberg SA, Morgan RA. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006;66:8878–8886. doi: 10.1158/0008-5472.CAN-06-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goff SL, Johnson LA, Black MA, Xu H, Zheng Z, Cohen CJ, Morgan RA, Rosenberg SA, Feldman SA. Enhanced receptor expression and in vitro effector function of a murine-human hybrid MART-1-reactive T cell receptor following a rapid expansion. Cancer Immunol Immunother. 2010;59:1551–1560. doi: 10.1007/s00262-010-0882-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuball J, Dossett ML, Wolfl M, Ho WY, Voss RH, Fowler C, Greenberg PD. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood. 2007;109:2331–2338. doi: 10.1182/blood-2006-05-023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen CJ, Li YF, El-Gamil M, Robbins PF, Rosenberg SA, Morgan RA. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 2007;67:3898–3903. doi: 10.1158/0008-5472.CAN-06-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voss RH, Willemsen RA, Kuball J, Grabowski M, Engel R, Intan RS, Guillaume P, Romero P, Huber C, Theobald M. Molecular design of the Calphabeta interface favors specific pairing of introduced TCRalphabeta in human T cells. J Immunol. 2008;180:391–401. doi: 10.4049/jimmunol.180.1.391. [DOI] [PubMed] [Google Scholar]
- 39.Sebestyen Z, Schooten E, Sals T, Zaldivar I, San Jose E, Alarcon B, Bobisse S, Rosato A, Szollosi J, Gratama JW, Willemsen RA, Debets R. Human TCR that incorporate CD3zeta induce highly preferred pairing between TCRalpha and beta chains following gene transfer. J Immunol. 2008;180:7736–7746. doi: 10.4049/jimmunol.180.11.7736. [DOI] [PubMed] [Google Scholar]
- 40.Roszik J, Sebestyen Z, Govers C, Guri Y, Szoor A, Palyi-Krekk Z, Vereb G, Nagy P, Szollosi J, Debets R. T-cell synapse formation depends on antigen recognition but not CD3 interaction: studies with TCR:ζ, a candidate transgene for TCR gene therapy. Eur J Immunol. 2011;41:1288–1297. doi: 10.1002/eji.200940233. [DOI] [PubMed] [Google Scholar]
- 41.Ochi T, Fujiwara H, Okamoto S, An J, Nagai K, Shirakata T, Mineno J, Kuzushima K, Shiku H, Yasukawa M. Novel adoptive T-cell immunotherapy using a WT1-specific TCR vector encoding silencers for endogenous TCRs shows marked antileukemia reactivity and safety. Blood. 2011;118:1495–1503. doi: 10.1182/blood-2011-02-337089. [DOI] [PubMed] [Google Scholar]
- 42.van der Veken LT, Coccoris M, Swart E, Falkenburg JH, Schumacher TN, Heemskerk MH. Alpha beta T cell receptor transfer to gamma delta T cells generates functional effector cells without mixed TCR dimers in vivo. J Immunol. 2009;182:164–170. doi: 10.4049/jimmunol.182.1.164. [DOI] [PubMed] [Google Scholar]
- 43.Sadelain M, Brentjens R, Rivière I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21:215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 45.Zhao Y, Wang QJ, Yang S, Kochenderfer JN, Zheng Z, Zhong X, Sadelain M, Eshhar Z, Rosenberg SA, Morgan RA. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J Immunol. 2009;183:5563–5574. doi: 10.4049/jimmunol.0900447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kowolik CM, Topp MS, Gonzalez S, Pfeiffer T, Olivares S, Gonzalez N, Smith DD, Forman SJ, Jensen MC, Cooper LJ. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66:10995–11004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 47.Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, Campana D. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 48.Wang J, Jensen M, Lin Y, Sui X, Chen E, Lindgren CG, Till B, Raubitschek A, Forman SJ, Qian X, James S, Greenberg P, Riddell S, Press OW. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum Gene Ther. 2007;18:712–725. doi: 10.1089/hum.2007.028. [DOI] [PubMed] [Google Scholar]
- 49.Charo J, Finkelstein SE, Grewal N, Restifo NP, Robbins PF, Rosenberg SA. Bcl-2 overexpression enhances tumor-specific T-cell survival. Cancer Res. 2005;65:2001–2008. doi: 10.1158/0008-5472.CAN-04-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eaton D, Gilham DE, O'Neill A, Hawkins RE. Retroviral transduction of human peripheral blood lymphocytes with Bcl-X(L) promotes in vitro lymphocyte survival in pro-apoptotic conditions. Gene Ther. 2002;9:527–535. doi: 10.1038/sj.gt.3301685. [DOI] [PubMed] [Google Scholar]
- 51.Dotti G, Savoldo B, Pule M, Straathof KC, Biagi E, Yvon E, Vigouroux S, Brenner MK, Rooney CM. Human cytotoxic T lymphocytes with reduced sensitivity to Fas-induced apoptosis. Blood. 2005;105:4677–4684. doi: 10.1182/blood-2004-08-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dagarag M, Evazyan T, Rao N, Effros RB. Genetic manipulation of telomerase in HIV-specific CD8+ T cells: enhanced antiviral functions accompany the increased proliferative potential and telomere length stabilization. J Immunol. 2004;173:6303–6311. doi: 10.4049/jimmunol.173.10.6303. [DOI] [PubMed] [Google Scholar]
- 53.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Foster AE, Dotti G, Lu A, Khalil M, Brenner MK, Heslop HE, Rooney CM, Bollard CM. Antitumor activity of EBV-specific T lymphocytes transduced with a dominant negative TGF-beta receptor. J Immunother. 2008;31:500–505. doi: 10.1097/CJI.0b013e318177092b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stephan MT, Ponomarev V, Brentjens RJ, Chang AH, Dobrenkov KV, Heller G, Sadelain M. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat Med. 2007;13:1440–1449. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- 56.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marktel S, Magnani Z, Ciceri F, Cazzaniga S, Riddell SR, Traversari C, Bordignon C, Bonini C. Immunologic potential of donor lymphocytes expressing a suicide gene for early immune reconstitution after hematopoietic T-cell-depleted stem cell transplantation. Blood. 2003;101:1290–1298. doi: 10.1182/blood-2002-08-2351. [DOI] [PubMed] [Google Scholar]
- 58.Mercier-Letondal P, Deschamps M, Sauce D, Certoux JM, Milpied N, Lioure B, Cahn JY, Deconinck E, Ferrand C, Tiberghien P, Robinet E. Early immune response against retrovirally transduced herpes simplex virus thymidine kinase-expressing gene-modified T cells coinfused with a T cell-depleted marrow graft: an altered immune response? Hum Gene Ther. 2008;19:937–950. doi: 10.1089/hum.2007.156. [DOI] [PubMed] [Google Scholar]
- 59.Traversari C, Marktel S, Magnani Z, Mangia P, Russo V, Ciceri F, Bonini C, Bordignon C. The potential immunogenicity of the TK suicide gene does not prevent full clinical benefit associated with the use of TK-transduced donor lymphocytes in HSCT for hematologic malignancies. Blood. 2007;109:4708–4715. doi: 10.1182/blood-2006-04-015230. [DOI] [PubMed] [Google Scholar]
- 60.Berger C, Flowers ME, Warren EH, Riddell SR. Analysis of transgene-specific immune responses that limit the in vivo persistence of adoptively transferred HSV-TK-modified donor T cells after allogeneic hematopoietic cell transplantation. Blood. 2006;107:2294–2302. doi: 10.1182/blood-2005-08-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deschamps M, Mercier-Lethondal P, Certoux JM, Henry C, Lioure B, Pagneux C, Cahn JY, Deconinck E, Robinet E, Tiberghien P, Ferrand C. Deletions within the HSV-tk transgene in long-lasting circulating gene-modified T cells infused with a hematopoietic graft. Blood. 2007;110:3842–3852. doi: 10.1182/blood-2007-04-087346. [DOI] [PubMed] [Google Scholar]
- 62.Sato T, Neschadim A, Konrad M, Fowler DH, Lavie A, Medin JA. Engineered human tmpk/AZT as a novel enzyme/prodrug axis for suicide gene therapy. Mol Ther. 2007;15:962–970. doi: 10.1038/mt.sj.6300122. [DOI] [PubMed] [Google Scholar]
- 63.Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, Straathof K, Liu E, Durett AG, Grilley B, Liu H, Cruz CR, Savoldo B, Gee AP, Schindler J, Krance RA, Heslop HE, Spencer DM, Rooney CM, Brenner MK. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomis DC, Marktel S, Bonini C, Traversari C, Gilman M, Bordignon C, Clackson T. A Fas-based suicide switch in human T cells for the treatment of graft-versus-host disease. Blood. 2001;97:1249–1257. doi: 10.1182/blood.v97.5.1249. [DOI] [PubMed] [Google Scholar]
- 65.Griffioen M, van Egmond EH, Kester MG, Willemze R, Falkenburg JH, Heemskerk MH. Retroviral transfer of human CD20 as a suicide gene for adoptive T-cell therapy. Haematologica. 2009;94:1316–1320. doi: 10.3324/haematol.2008.001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoon SH, Lee JM, Cho HI, Kim EK, Kim HS, Park MY, Kim TG. Adoptive immunotherapy using human peripheral blood lymphocytes transferred with RNA encoding Her-2/neu-specific chimeric immune receptor in ovarian cancer xenograft model. Cancer Gene Ther. 2009;16:489–497. doi: 10.1038/cgt.2008.98. [DOI] [PubMed] [Google Scholar]
- 67.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, Duray PH, Steinberg SM, Allison JP, Davis TA, Rosenberg SA. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meijer SL, Dols A, Jensen SM, Hu HM, Miller W, Walker E, Romero P, Fox BA, Urba WJ. Induction of circulating tumor-reactive CD8+ T cells after vaccination of melanoma patients with the gp100 209-2M peptide. J Immunother. 2007;30:533–543. doi: 10.1097/CJI.0b013e3180335b5e. [DOI] [PubMed] [Google Scholar]
- 69.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, Restifo NP. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cooper LJ, Al-Kadhimi Z, Serrano LM, Pfeiffer T, Olivares S, Castro A, Chang WC, Gonzalez S, Smith D, Forman SJ, Jensen MC. Enhanced antilymphoma efficacy of CD19-redirected influenza MP1-specific CTLs by cotransfer of T cells modified to present influenza MP1. Blood. 2005;105:1622–1631. doi: 10.1182/blood-2004-03-1208. [DOI] [PubMed] [Google Scholar]
- 71.Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, Palmer DC, Boni A, Muranski P, Yu Z, Gattinoni L, Antony PA, Rosenberg SA, Restifo NP. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 73.Choi D, Kim KS, Yang SH, Chung DH, Song B, Sprent J, Cho JH, Sung YC. Dendritic cell internalization of α-galactosylceramide from CD8 T cells induces potent antitumor CD8 T-cell responses. Cancer Res. 2011;71:7442–7451. doi: 10.1158/0008-5472.CAN-11-1459. [DOI] [PubMed] [Google Scholar]
- 74.Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the 'Swiss-Army knife' of the immune system. Curr Opin Immunol. 2008;20:358–368. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou D, Cantu C, 3rd, Sagiv Y, Schrantz N, Kulkarni AB, Qi X, Mahuran DJ, Morales CR, Grabowski GA, Benlagha K, Savage P, Bendelac A, Teyton L. Editing of CD1d-bound lipid antigens by endosomal lipid transfer proteins. Science. 2004;303:523–527. doi: 10.1126/science.1092009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kang SJ, Cresswell P. Saposins facilitate CD1d-restricted presentation of an exogenous lipid antigen to T cells. Nat Immunol. 2004;5:175–181. doi: 10.1038/ni1034. [DOI] [PubMed] [Google Scholar]
- 77.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]