Abstract
Although metastasis is relatively frequent in cases of renal cell carcinoma (RCC), metastasis in the cervical or supraclavicular lymph node (LN) is relatively rare. Moreover, cases of metastatic RCC with a non-identifiable kidney mass are extremely rare. Here, the authors report a case of metastatic RCC in a supraclavicular LN without a primary kidney lesion. A 69-year-old man presented with a progressively enlarging right supraclavicular mass. Incisional biopsy of the affected supraclavicular LN was performed, and histological examination revealed metastatic RCC. However, no tumor was found in either kidney, despite various examinations. The patient was treated with radiotherapy followed by sunitinib. After three months on sunitinib, a follow-up computed tomography scan revealed that the supraclavicular LN had markedly decreased, and after 20 months, the disease had not progressed. This case suggests that, even when there is no primary kidney lesion, clinicians must consider the possibility of metastatic RCC when evaluating patients with clear cell carcinoma with an unknown primary site.
Keywords: Lymph nodes, Metastasis, Renal cell carcinoma
Introduction
Renal cell carcinoma (RCC) can metastasize to any location in the body, and distant metastases are common, being present in 30% to 40% of patients with metastatic disease [1]. The most common sites affected are the lungs (76%), regional lymph nodes (LN) (66%), bone (42%), and liver (41%) [2]. Furthermore, approximately 15% of RCC patients have extracranial head and neck metastases [3]. However, only 1.5 to 3.5% of RCC patients show solitary metastasis, and only 1% have metastasis confined to the head and neck [3]. Moreover, cases of metastatic RCC with a non-identifiable kidney mass are extremely rare. Here, we report a case of metastatic RCC in only a supraclavicular LN with no evidence of a primary kidney lesion.
Case Report
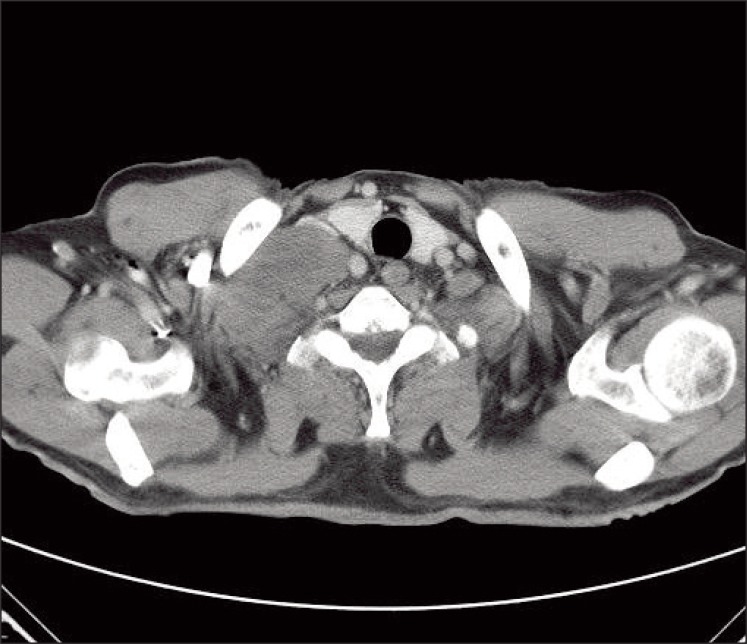
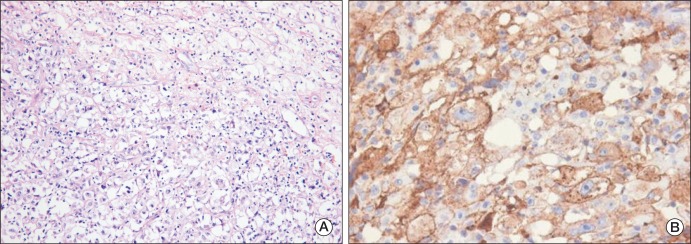
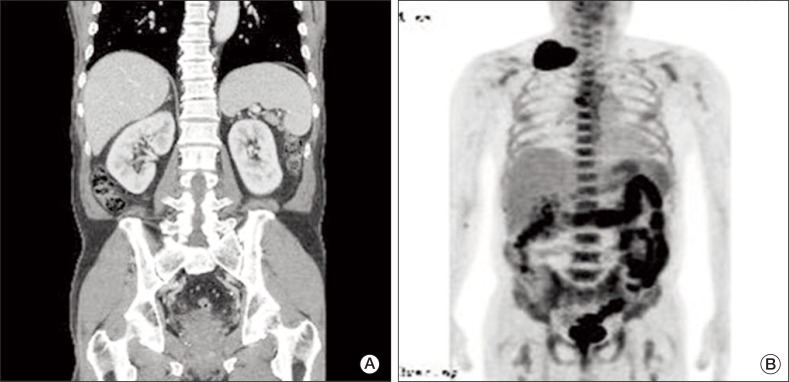
A 69-year-old man presented with a 2-week history of a progressively enlarging supraclavicular mass in April 2009. He had no relevant previous medical history. Written consent was obtained from the patient for publication of the study. Upon physical examination, his body temperature was 37.3℃, blood pressure 130/90 mmHg, and pulse rate 75/min. A head and neck examination revealed a palpable firm, fixed, non-tender, 8 cm-sized mass in the right supraclavicular area with no other evident lymphadenopathy, and an abdominal examination revealed no palpable mass. His complete blood cell count, chemistry findings, and renal functionwere normal. Urine dipstick and microscopy tests revealed no hematuria or abnormal cells. Computed tomography (CT) of the neck showed right supraclavicular LN enlargement (Fig. 1). An incisional biopsy was performed on the supraclavicular LN. Hematoxylin and eosin staining revealed that tumor cells were polygonal with clear cytoplasm and vesicular nuclei with prominent nucleoli (Fig. 2A). Immunohistochemical staining showed that tumor cells were positive for pancytokeratin (1 : 300, Leica, Newcastle upon Tyne, UK), vimentin (1 : 200, Dako, Glostrup, Demark), and CD10 (1 : 100, Leica) (Fig. 2B), but negative for thyroid transcription factor 1 (1 : 300, Leica), cytokeratin 7 (1 : 600, NeoMarkers, Fremont, CA), cytokeratin 20 (1 : 150, Dako) and hepatocyte specific antigen (1 : 50, Leica). Based on these findings, a diagnosis of metastatic clear cell RCC was made, after which the patient underwent abdominal/pelvic CT (Fig. 3A) and a positron emission tomography-CT scan (Fig. 3B). The results revealed no tumor in either kidney, and no further metastatic lesions; accordingly, he was diagnosed as having metastatic RCC in a supraclavicular LN only, without an identifiable kidney mass.
Fig. 1.
Computed tomography of the neck showing lymph node enlargement in the right supraclavicular area.
Fig. 2.
Pathological features of an incisional biopsy of the supraclavicular lymph node. (A) The tumor cells had clear cytoplasm and vesicular nuclei with prominent nucleoli, and were are arranged in nests admixed with intervening blood vessels (H&E staining, ×200). (B) Immunohistochemistry showed that the tumor cells were positive for CD10 (×400).
Fig. 3.
Computed tomography (CT) of the abdomen/pelvis and positron emission tomography (PET)-CT. (A) CT of the abdomen and pelvis revealing no tumor in either kidney. (B) PET-CT images show the right supraclavicular lymph node with 18-fluoro-deoxyglucose (FDG) uptake (standardized uptake value, SUV, 14.7), compatible with malignant lymphadenopathy. However, no other FDG uptake was found in either kidney, and there were no additional metastatic lesions.
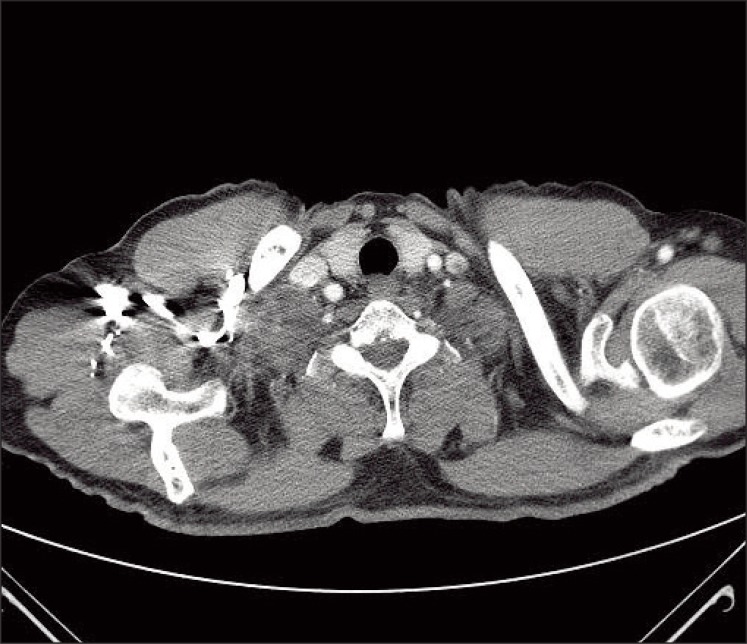
The patient was initially treated with radiotherapy (4,000 cGy administered in 15 fractions over a period of 3 weeks), which induced tumor shrinkage, after which he was administered sunitinib (50 mg/day consecutively for four weeks followed by two weeks off per cycle). After two cycles, a follow-up CT scan revealed that the supraclavicular LN had markedly decreased in size (Fig. 4). Upon completing two cycles, the acute toxicity (grade 3 hand-foot syndrome, asthenia, and grade 2 thrombocytopenia) led to dose reduction (25 mg/day consecutively for four weeks followed by two weeks off). After 20 months of sunitinib under the reduced dose, no tumor progression was observed. The patient continues to be followed and undergoes regular urological reviews. Repeated follow-up abdominal/pelvic CT for 20 months has revealed no evidence of a primary renal tumor.
Fig. 4.
Computed tomography of the neck after radiotherapy and two cycles of sunitinib showing markedly reduced lymph node enlargement in the right supraclavicular area.
Discussion
The most common metastatic sites of RCC are the lungs, regional LN, bone, and liver [2], and it is uncommon for RCC to present a solitary metastasis at an unusual site. The mechanism of metastasis to unusual sites has not been clearly explained, but the mechanism of solitary metastasis of RCC to the head and neck region (an unusual site) is explained as follows. Batson's paraspinal plexus is a rich, valve-less venous anastomosis with prevertebral, vertebral, and epidural systems [4], and this rich venous system offers little resistance to the spread of tumor emboli. Accordingly, the vertebral venous plexus offers a means of bypassing the pulmonary venous system and facilitates metastasis in the head and neck region without lung involvement [3,5]. Metastases that do not follow normal hematogenous flow patterns may also be explained by a right-to-left heart shunt, spontaneous regression of lung disease, or microscopic seeding of lung parenchyma [3].
Moreover, non-identifiable kidney lesions are extremely rare in metastatic RCC. In such cases, the disease presents as carcinoma of an unknown primary form with a clear cell neoplasm. However, diagnostic pathology has improved remarkably, and the routine use of electron microscopy and immunohistochemistry and the emerging field of molecular genetics are enabling more precise diagnoses. Differential diagnoses in malignant neoplasms composed of clear cells include hepatocellular carcinoma, adrenal cortical carcinoma and clear RCC. Hepatocellular carcinoma is positive for cytokeratin, epithelial membrane antigen (epithelial markers), and hepatocyte specific antigen, but negative for vimentin, whereas adrenal cortical carcinoma is positive for vimentin, but negative for epithelial markers. RCC is positive for epithelial markers, vimentin, and CD10, similar to the neoplastic cells in our case. A number of previous case reports have also described cases of metastatic clear cell renal carcinoma without a primary renal mass [6,7]. In the case of metastatic RCC reported by Kawakita et al. [6], no renal tumor was observed initially, but a renal tumor did appear after two years [6]. Bhatia et al. [7] also reported a case of cutaneous facial RCC with no known primary tumor. It is not known why the primary lesion remains occult, but it may be involved or too small to be detected. Nevertheless, these lesions could be silent renal tumors; therefore, close monitoring is essential.
As in our case, patients with single site involvement (brain, liver, subcutaneous tissue, bone, intestine, LN, or others) should be treated using aggressive local therapy such as resection, radiation therapy, or both, because a minority enjoy long-term, disease-free survival [3,8]. In addition to definite local therapy, these patients should also receive systemic therapy based on one of the newer regimens because they seem to confer better survival. In our case of solitary metastasis in a supraclavicular LN, radiation therapy was applied and systemic therapy was performed with sunitinib, which is a standard treatment for metastatic RCC. As a result, the patient has been well for the last 20 months without disease progression. Sunitinib is a multitargeting receptor tyrosine kinase inhibitor of vascular endothelial growth factor and of platelet-derived growth factor receptor [9]. In a recent phase III trial, progression-free survival was found to be longer and quality of life to be better for patients with metastatic RCC treated with sunitinib than for those treated with interferon-α [10].
Not infrequently, clinicians and pathologists encounter malignant neoplasms composed of clear cells that have sources and natures that cannot be determined by conventional morphological studies. Therefore, immunocytochemical staining for cytokeratin, epithelial membrane antigen, vimentin, and fat are of considerable diagnostic help in RCC. This case report suggests that even in the absence of a primary kidney lesion, clinicians must consider the possibility of metastatic RCC when evaluating patients with clear cell carcinoma from an unknown primary site. Furthermore, if only one metastatic site is present, a local modality such as surgical resection or radiotherapy should be applied, and if necessary, systemic treatment like sunitinib added.
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (No. 2012-0000475).
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Golimbu M, Joshi P, Sperber A, Tessler A, Al-Askari S, Morales P. Renal cell carcinoma: survival and prognostic factors. Urology. 1986;27:291–301. doi: 10.1016/0090-4295(86)90300-6. [DOI] [PubMed] [Google Scholar]
- 2.Maldazys JD, deKernion JB. Prognostic factors in metastatic renal carcinoma. J Urol. 1986;136:376–379. doi: 10.1016/s0022-5347(17)44873-7. [DOI] [PubMed] [Google Scholar]
- 3.Pritchyk KM, Schiff BA, Newkirk KA, Krowiak E, Deeb ZE. Metastatic renal cell carcinoma to the head and neck. Laryngoscope. 2002;112:1598–1602. doi: 10.1097/00005537-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Cheng ET, Greene D, Koch RJ. Metastatic renal cell carcinoma to the nose. Otolaryngol Head Neck Surg. 2000;122:464. doi: 10.1016/S0194-5998(00)70069-6. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto R, Helmus C. Hypernephroma metastatic to the head and neck. Laryngoscope. 1973;83:898–905. doi: 10.1288/00005537-197306000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Kawakita M, Kawamura J, Hida S, Ooishi K, Okada K, Yoshida O. Renal cell carcinoma, primary lesion which was not easily identified: report of two cases. Hinyokika Kiyo. 1985;31:463–473. [PubMed] [Google Scholar]
- 7.Bhatia S, Ng S, Hodder SC. Metastatic cutaneous head and neck renal cell carcinoma with no known primary: case report. Br J Oral Maxillofac Surg. 2010;48:214–215. doi: 10.1016/j.bjoms.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Rasco DW, Assikis V, Marshall F. Integrating metastasectomy in the management of advanced urological malignancies-where are we in 2005? J Urol. 2006;176:1921–1926. doi: 10.1016/j.juro.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 10.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]