Abstract
Liver injury due to prescription and nonprescription medications is a growing medical, scientific, and public health problem. Worldwide, the estimated annual incidence rate of drug-induced liver injury (DILI) is 13.9-24.0 per 100,000 inhabitants. DILI is one of the leading causes of acute liver failure in the US. In Korea, the annual extrapolated incidence of cases hospitalized at university hospital is 12/100,000 persons/year. Most cases of DILI are the result of idiosyncratic metabolic responses or unexpected reactions to medication. There is marked geographic variation in relevant agents; antibiotics, anticonvulsants, and psychotropic drugs are the most common offending agents in the West, whereas in Asia, 'herbs' and 'health foods or dietary supplements' are more common. Different medical circumstances also cause discrepancy in definition and classification of DILI between West and Asia. In the concern of causality assessment, the application of the Roussel Uclaf Causality Assessment Method (RUCAM) scale frequently undercounts the cases caused by 'herbs' due to a lack of previous information and incompatible time criteria. Therefore, a more objective and reproducible tool that could be used for the diagnosis of DILI caused by 'herbs' is needed in Asia. In addition, a reporting system similar to the Drug-Induced Liver Injury Network (DILIN) in the US should be established as soon as possible in Asia.
Keywords: Drug-Induced Liver Injury, Asia, Herbal Medicine
INTRODUCTION
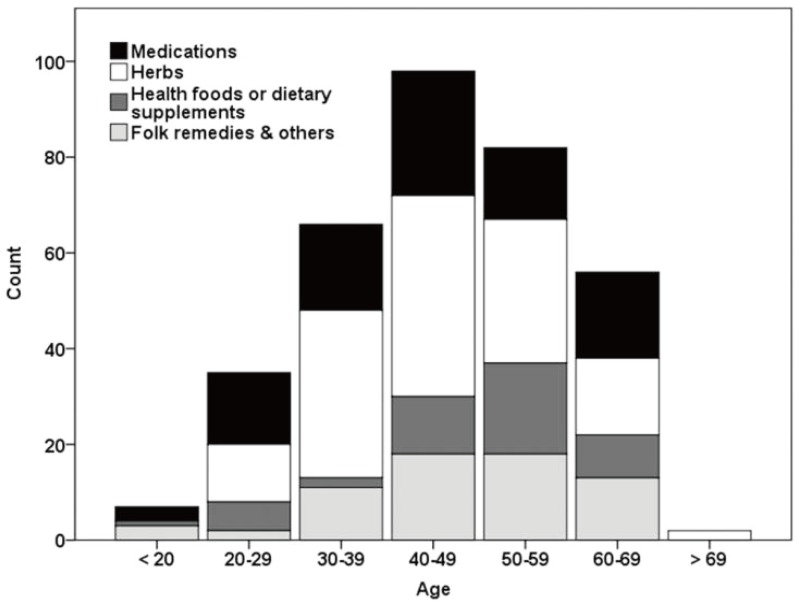
Drug-induced liver injury (DILI) is defined as a liver injury caused by various medications, herbs, or other xenobiotics, leading to abnormalities in liver tests or liver dysfunction with the reasonable exclusion of other etiologies.1 DILI is one of the leading causes of acute liver failure in the US, accounting for 13% of cases of acute liver failure; these events pose a major challenge for drug development and safety.2-7 Antimicrobials and agents for the central nervous system are the most common causes of DILI and health foods or dietary supplements account for 7% of cases of DILI in the US.1,3 In Korea, the annual extrapolated incidence of hospitalized cases at university hospital was calculated to be 12/100,000 persons/year.8 The age distribution was varied, with the age groups <20, 20-29, 30-39, 40-49, 50-59, and ≥60 representing 1.3%, 8.1%, 16.4%, 27.5%, 21.8%, and 24.8% of cases, respectively. There was no significant difference in etiology between age groups (Fig. 1).8
Figure 1.
Incidence of drug-induced liver injury according to age.8
The medical milieu of Korea provides an especially interesting case in the study of DILI because Oriental Medicine has been widely accepted as an alternative to modern medicine. Moreover, many such 'folk remedies', assumed to be safe and natural, are often employed without any regulation or expert advice in the treatment of various conditions. Therefore, many Koreans are often exposed to 'herbs' and 'folk remedies' that may lead to DILI; a similar situation is found in other Asian countries. 'Herbal medications' are the principal cause of DILI in Korea.8,9 These statistics highlight the relevance of DILI as one of major health problems in Korea. Unfortunately, in Korea and other Asian countries, there are limited prospective data regarding the epidemiology and clinical course of DILI, as well as the social burden and mortality of patients with this condition.
Pathogenesis of DILI
Medications can cause a diverse array of acute or chronic liver injury.5 Most cases of DILI are the result of idiosyncratic metabolic responses or unexpected reactions to medication, although the precise pathogenesis of such events is poorly understood.5,10-12 Idiosyncrasy implies the unusual presence of one or several factors that contribute to the development of DILI in an individual patient. Recent progress in research on DILI has been determined by key developments in two areas. First, new technologies allow the identification of genetic risk factors. Second, new mechanistic concepts of DILI emphasize the importance of unspecific 'downstream' events following drug-specific initial 'upstream' hepatocyte injury and of complex interactions between environmental and genetic risk factors.13,14
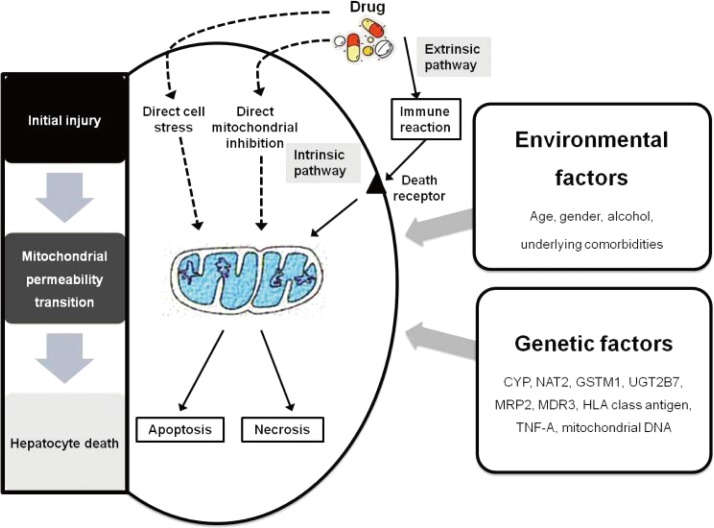
A three-step working model of DILI mechanisms has been suggested.15 According to this model, drugs or their metabolites first cause direct cell stress (intrinsic pathway), trigger immune reactions (extrinsic pathway), and/or directly impair mitochondrial function. Second, this 'initial hit' may lead to mitochondrial permeability transition, which in a third and final step can initiate apoptotic or necrotic cell death, depending on the availability of adenosine triphosphate.15 Several amplification mechanisms are highlighted and may play important roles in the idiosyncratic occurrence of DILI. The three-step mechanistic working model may be a simplification of a more complex reality, needing further modification with increasing insight into mechanisms of DILI, but it currently provides a helpful framework for understanding the complex interactions between genetic and environmental risk factors that modulate the fragile balance between injurious and protective processes and therefore determine the occurrence and outcome of DILI (Fig. 2).16-18
Figure 2.
Three-step mechanism of drug-induced liver injury.
The liver removes lipophilic chemicals, including drugs, and biotransforms them into water-soluble metabolites which are then excreted. This process involves cytochrome P450 (phase 1), conjugation (phase 2) and transport (phase 3). The expression of the enzymes and transporters involved in hepatic handling of drug are under the control of transcription factors such as pregnane X receptor and constitutive androstane receptor. In addition, polymorphisms of these phase 1, 2 and 3 genes and transcription factors affect their activities and expression in response to environmental factors.
Following exposure, the toxic moiety induces a type of stress or functional disturbance. Mitochondria have emerged as one of the most important targets of this disturbance. Mice which are heterozygous for knockout of superoxide dismutase 2, the mitochondrial form of superoxide dismutase which protects against oxidative stress, developed liver injury after 4 weeks of troglitazone administration.19 Cellular necrosis depends upon the rapid loss of mitochondrial function, whereas in the superoxide dismutase positive model, troglitazone induces a more delayed loss of mitochondrial function. This is best understood as a threshold phenomenon in which mitochondria have a large reserve. When sufficient loss of mitochondrial DNA occurs, or modification of mitochondrial electron transport proteins accumulates, oxidative stress from increased reactive oxygen species overwhelms the antioxidant defence of mitochondria.
An important concept in DILI is adaptation. This is a situation in which the injury reverses with the continuation of the drug. A number of responses could mediate adaptation. Alterations in phases 1, 2 or 3 could dampen the exposure of hepatocytes to the toxic chemical. Oxidative stress induced by the toxic chemical or its effects on mitochondria can activate nuclear factor erythroid 2-related factor, a transcription factor which activates the expression of antioxidant genes.20 Mitochondrial damage induces mitochondrial biogenesis, and endoplasmic reticulum stress induces an adaptive response to modulate stress.21
Diagnosis of DILI
The diagnosis of DILI can be difficult due to the lack of specific signs, symptoms and tests and is, in part, a diagnosis based on exclusion. The manifestations of drug hepatotoxicity are highly variable, ranging from asymptomatic elevation of liver enzymes to fulminant hepatic failure. Thus, diagnosis must rely on comprehensive clinical assessment. Typically, the clinical history indicates a suspect drug with reasonable temporal association to the illness. A pattern of liver injury which is characteristic of the drug is also helpful in diagnosis. Finally, other causes of acute liver injury must be excluded.
One of the most demanding and challenging issues of DILI is the attribution and assessment of causality. There are three approaches to determine attribution of causality.22-24 Foremost, a positive rechallenge which fulfills the Koch's postulate is regarded as the gold standard. However, this approach is clinically limited since it is often unacceptably dangerous. The second method, the ad hoc approach, appears to be reasonable at the first glance but has no solid logical justification. The third and the most widely used method is the Roussel Uclaf Causality Assessment Method (RUCAM) scale.
The RUCAM scale has been validated in several studies. It is intended to provide objective and consistent assessment, but can be cumbersome for routine clinical use. This method, when first validated, demonstrated 86% sensitivity, 89% specificity and positive and negative predictive values of 93% and 78%, respectively, using a cut-off point of 5.25 The reproducibility of the RUCAM scale was evaluated by its application to 50 suspected DILI cases by four experts. Agreement between two, three, and four experts was 99%, 74%, and 37%, respectively, indicating perturbing discrepancies as the number of applications of the scale increases.26 In the United States, The Drug-Induced Liver Injury Network (DILIN) was initiated in 2004 to register patients with well characterized liver injury presumed to be due to 'medications' or 'herbal and dietary supplements'. Clinical data as well as biological samples are collected for this multicenter database project. The DILIN employs a combination of expert opinion and the RUCAM scale to assess DILI causality. However, a recent comparison revealed a weak correlation between expert opinion and the RUCAM scale.27 Therefore, although expert opinion appeared to be the better causality assessment tool, there was still need for a simple, accurate and reproducible method of diagnosing DILI.
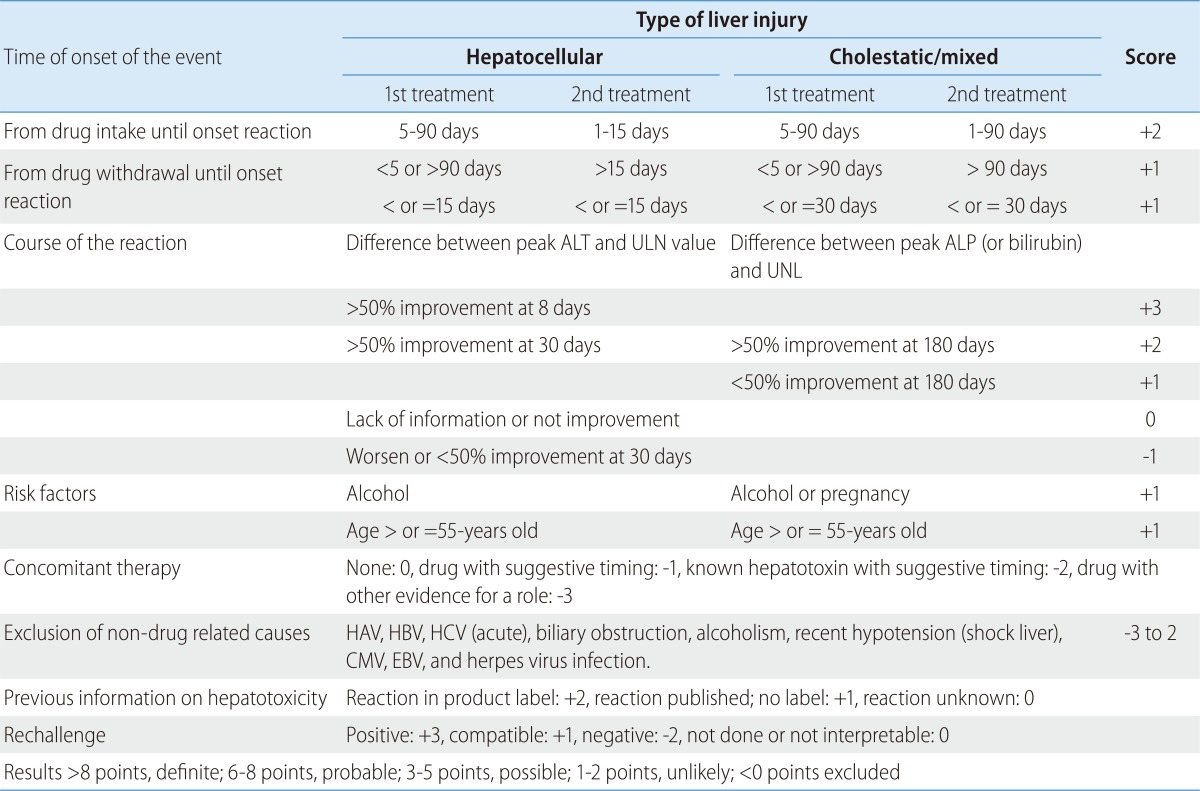
Specifically, the RUCAM scale provides a semi-quantitative evaluation of causality through assigning a score (ranging between -3 and +3 points) to each of its seven domains (time to onset of the reaction from both the beginning and cessation of use of the causative agents; course of the reaction; risk factors; concomitant medications; non-medication related causes; previous information on the medication; and response to re-administration if any). Theoretically, the overall score may range anywhere from -5 to +14. Based on the final score, a causal relationship between the implicated agent and the liver injury event was established as highly probable (>8), probable (6-8), possible (3-5), unlikely (1-2), or excluded (<0) (Table 1).25,26
Table 1.
ALT, alanine aminotransferase; UNL, upper normal limit; ALP, alkaline phosphatase.
In 1997, Maria and Victorino (M&V) developed a simplified scoring system in an attempt to overcome the complexity of the RUCAM scale. This system, referred to as the Clinical Diagnostic Scale or the M&V scale, uses several features of the RUCAM scale, but focuses on less components.28 The overall score corresponds to five probability degrees: definite, probable, possible, unlikely, and excluded. The M&V scale was validated using real and fictitious cases, and was compared with the classification of three external experts. The comparison showed 84% agreement between the scale and expert opinions.28 Nevertheless, the M&V scale has some limitations. The scale classifies cases as definite only when 'positive rechallenge' and hypersensitivity features are present, despite these features being comparatively infrequent suspected cases of DILI.29 Besides, the instrument performs poorly in atypical cases, such as those with unusually long latency periods or those leading to chronic evolution after drug withdrawal.30 In addition, drugs which have been on the market for more than five years, and those with no documented hepatotoxicity potential are given a lower score and criteria for exclusion of alternative causes are poorly described. Taken together, these limitations make it difficult to generate high scores for most hepatic reactions and to ascertain a diagnosis of DILI with confidence.
A new diagnostic scale, the Digestive Disease Week-Japan (DDW-J) scale, was recently proposed in Japan. This scale was derived from the RUCAM scale, but with modifications in the items concerning chronologic criteria, concomitant drug, and extrahepatic manifestations.31 The DDW-J scale has been shown to accurately diagnose DILI and to be superior to the RUCAM and M&V scale.32 The DDW-J scale includes an in vitro drug lymphocyte stimulation test (DLST) evaluation criterion. Limited access and lack of standardization have prevented generalized clinical use of the DLST outside of Japan, and consequently have reduced DDW-J scale applications. However, recent findings of specific HLA (Human Leucocyte Antigen) allele associations with DILI, suggesting an important role for the adaptive immune response in DILI pathogenesis, have highlighted the potential value of DLST's, and promoted a new interest for lymphocyte-based tests in DILI assessments.33
R values: type of DILI
The initial step when applying the RUCAM scale is to define the type of liver injury based on the pattern of serum enzymes by calculating the R value. For the clinical characterization of DILI, the ratio of serum alanine aminotransferase (ALT) to alkaline phosphatase (ALP) was designated as the R value ([ALT value/ALT upper normal limit (UNL)]/[ALP value/ALP UNL]).34 Hepatocellular DILI was defined as R≥5, cholestatic as R≤2, and mixed as 2<R<5.35
Depending on the pattern of liver damage, some items in the scale will score differently. Importantly, mixed types of damage fall into the cholestatic damage category although no data support strict resemblance in disease manifestation, severity, or progression. Furthermore, the type of liver injury may change during the course of the illness.36 Due to variations in the serum enzyme profile during disease progression, the time point for calculating the R value is important. Many clinicians use enzyme values from the first analytical test showing elevations above normal to establish the R value, while others use peak values, which may or may not coincide with the initial analytical values. The lack of clear instructions for how the R value should be calculated can lead to differences among users in defining the type of liver injury.
Classification of DILI
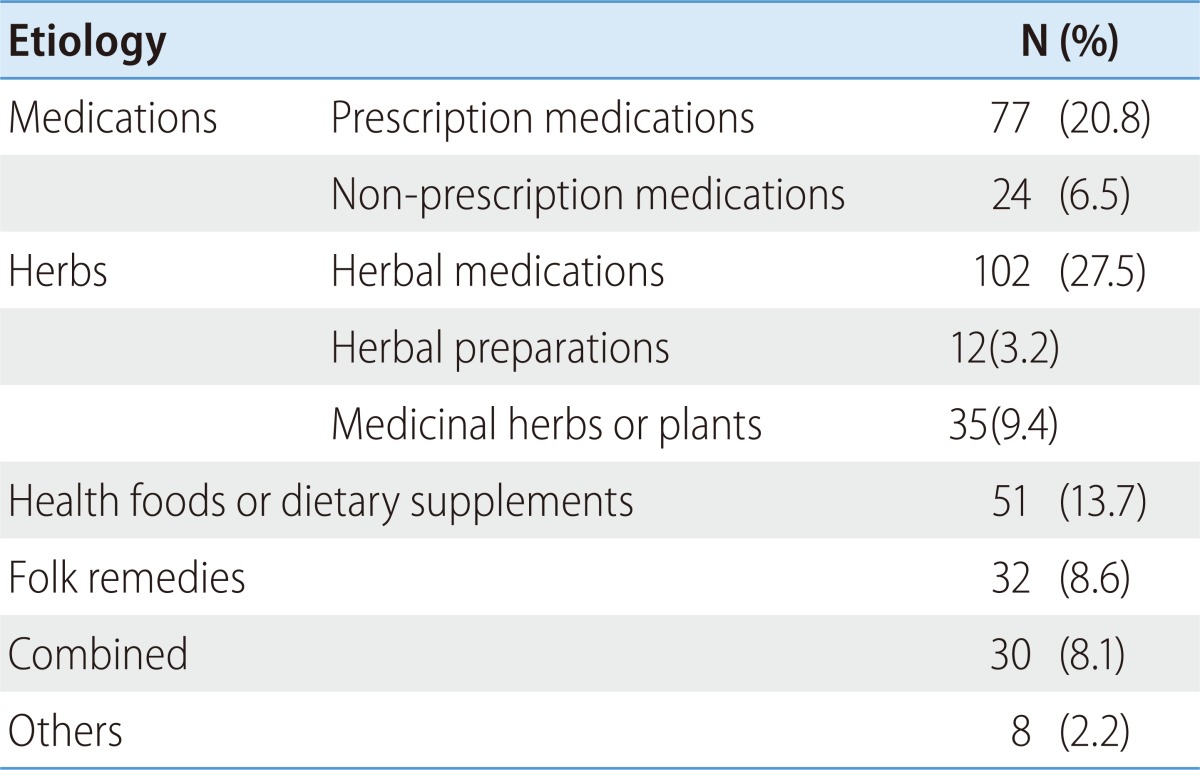
DILI can be classified according to causative agent using these categories: 'medications', 'herbs', 'health foods or dietary supplements', 'folk remedies', 'combined', and 'others'. 'medications' can be sub-classified as either 'prescription medications' (medications which are prescribed by a medical doctor) or 'non-prescription medications' (over the counter medications which are bought without prescription). 'Herbs' can be sub-categorized as 'herbal medications' (medications prescribed and compounded by a doctor of Oriental Medicine), 'herbal preparations' (preparations compounded by an Oriental Pharmacist), or 'medicinal herbs or plants" (preparation compounded by an unauthorized layperson). Other traditional remedies that do not fit any of the previous categories ('herbal medications' and 'herbal preparations') can be classified as 'folk remedies'. Preparations intended to supplement the diet and provide nutrients (vitamins, minerals, fiber, fatty acids, amino acids, et cetera) that may be missing or may not be consumed in sufficient quantities in a person's diet can be classified as 'health foods or dietary supplements'.
In Korea, the most common causative agent identified was 'herbal medications' (27.5%). 'Prescription medications' was implicated in 20.8% of patients while 'health foods or dietary supplements' were linked in 13.7% of patients. Other causes were 'medicinal herbs or plants' (9.4%), 'folk remedies' (8.6%), 'combined' (two or more causative agents) (8.1%), 'non-prescription medications' (6.5%), 'herbal preparations' (3.2%), and others (2.2%) (Table 2).8 An herbal decoction was found to be the most common form of etiology in Korea. Among medications, an antifungal agent was found to be the most common cause of DILI (Table 2).8
Table 2.
Classification of causative agents8
N, number.
Treatment of DILI
DILI has a wide spectrum of manifestations, ranging from asymptomatic mild biochemical abnormalities to severe hepatitis with jaundice. In most cases of DILI, liver injury is expected to improve following discontinuation of the suspected drug. On the other hand, some DILI patients show resolution of liver injury without discontinuation of the drug. Therefore, it should be carefully evaluated whether the suspected drug should be discontinued, in consideration of the importance of the medication and the degree of damage being caused by its administration.37
Clinical jaundice as a result of administered drugs was associated with poor prognosis (a fatality rate of 10%) for many drugs.38 Zimmerman reported that elevation of transaminase activities in combination with jaundice suggests serious liver injury with fatalities.39-41 These findings were discussed at the National Institutes of Health (NIH), and are recognized as Hy's rule for monitoring DILI, which states that elevation of liver enzymes (AST or ALT more than 3¡¿ULN or ALP more than 1.5×ULN) in combination with elevated bilirubin (more than 3×ULN) at any time after starting a new drug may imply serious liver injury and it is recommended that treatment with the suspected drug be stopped. Two recent studies have shown that hepatocellular liver injury with jaundice is sometimes fatal even when the suspected drug is stopped.29,42 On the other hand, a recent study showed that cases fulfilling Hy's rule did not always lead to death from DILI.43
As many drugs can induce asymptomatic elevation of liver enzyme levels without severe hepatotoxicity, mild elevations in transaminases do not always require withdrawal of the causative drug. Based on these observations, the FDA (Food and Drug Administration) recently proposed draft guidelines in which ALT greater than 8×ULN, ALT greater than 5×ULN for two weeks, ALT greater than 3×ULN in association with serum bilirubin greater than 2×ULN, more than 1.5×PT-INR, or symptoms of liver injury should be used to predict severe hepatotoxicity and recommend discontinuing the drug.4 Hepatocellular liver injury with severe jaundice should be treated carefully, and requires prompt referral to a hepatologist. As mentioned above, severe liver injury and fatality occur in cases of hepatocellular injury with jaundice.
There have been no reports of beneficial therapies, other than the use of N-acetylcysteine for acetaminophen hepatotoxicity.44 Corticosteroid therapy may be used in DILI cases with evident hypersensitivity, but it does not have proven benefits. Management of DILI is centered on the prompt withdrawal of the suspected drug. A positive re-challenge is a 50% decrease in serum ALT within 8 days of discontinuation of the suspected drug in the hepatocellular type, which is also included in the RUCAM scale.27 On the other hand, improvement of biliary enzymes after cessation of the suspected drug usually requires a longer period in the cholestatic type.
Prognosis of patients with DILI
The majority of patients with symptomatic acute DILI are expected to completely recover with supportive care after discontinuation of the suspect drug. In addition, patients with milder, and possibly unrecognizable episodes of DILI are also expected to recover without residual clinical, laboratory, radiological, or histological evidence of liver disease.45 The majority of DILI patients with clinically significant liver injury such as jaundice also have a generally favorable prognosis for recovery. For example, 712 of 784 (90.8%) DILI patients with jaundice recovered, and only 72 (9.2%) died or underwent liver transplant surgery.45 In contrast, the prognosis of patients with severe DILI who progress to acute liver failure with concomitant coagulopathy and encephalopathy is usually poor.46,47 Although DILI is more common in males, more females developed fulminant hepatic failure.
In most Western countries, acetaminophen overdose is the most frequently identified etiology of acute liver failure. However, there were a few cases (2%) caused by acetaminophen in Korea.8 Fortunately, prognosis is generally better in cases of acetaminophen-induced liver failure patients treated with N-acetylcysteine than in patients with other causes of DILI (60 to 80% versus 20 to 40%).48 In the prospective study, advanced coma grade at admission was associated with a poor survival in all subgroups, whereas patient age, sex, and ethnicity were not. Empiric use of corticosteroids in acute liver failure due to DILI is not recommended due to the lack of benefit in previously reported studies.49 Overall, it is recommended to refer patients with severe DILI to a transplant center in light of their potential for a poor outcome. Model for End-Stage Liver Disease score may be useful as a predictor of prognosis in patients admitted with acetaminophen toxicity.50
A small proportion of patients may develop chronic liver disease. A prospective study of DILI patients registered in the Spanish hepatotoxicity registry revealed a 5.7% incidence of chronic DILI.36 Recently, 6 months after enrollment in the prospective DILIN study, 14% of patients had persistent laboratory abnormalities.51 There were few deaths or transplantations (1.8%) cases of DILI in the Korean study,8 a finding that is distinct from prior reports that suggested that 5.6-17.3% of DILI cases had died or required transplantation,2,29,45,52,53 possibly indicating a reduced severity in cases of DILI resulting from 'herbs'. It was also found that herbs tended to cause DILI more frequently among female patients in Korea. These findings suggest that DILI might be affected by racial, ethnic, or sexual differences. Further studies are required in order to fully explore this hypothesis.
Unresolved issues of DILI
Definition of DILI
There is increasing concern about the potential risk of DILI from complementary and alternative medicines (CAM) including herbal products because they are unregulated and therefore not standardized with regard to their contents. CAM, including 'herbs' and 'health foods or dietary supplements' seems to be major causes of DILI in Asian countries. Moreover, with the widespread use of CAM, DILI from CAM seems to now be a worldwide problem.
Recently, 'herbs' and 'health foods or dietary supplements' have been included in the definition of DILI. However, the classification and definition of the causative agents can be difficult because of ambiguous boundaries. 'Herbs' might be sub-categorized into 'herbal medications' (medications prescribed and compounded by a doctor of Oriental Medicine), 'herbal preparations' (preparations compounded by an oriental pharmacist), and 'medicinal herbs or plants' (preparation compounded by an unauthorized lay person). However, 'drug' in definition DILI commonly means liver damage by prescriptional and over-the-counter drugs. Therefore, in Korea, DILI may not be appropriate because "medicinal herbs or plants" may be prescribed in the market by an unauthorized lay person. For this reason, toxic liver injury might be a more proper terminology within Korea. However, since toxic liver injury is not a Medical Subject Headings (MeSH) term, for the academic research and development, use of DILI is preferable.
In addition, there is no uniformity of laws and ordinances in Korea. The pharmaceutical law controls 'medications', 'herbal medications', and 'herbal preparations'. 'Medicinal herbs or plants' is defined in the 'herbal quality and distribution management standard' of the Ministry of Health and Welfare. Therefore, clinicians cannot clearly classify the same ingredients prescribed by doctors of Oriental Medicine, pharmacists, Oriental Pharmacists, or unauthorized lay persons.
Causality assessment
Identifying DILI remains a major challenge in clinical practice due to lack of reliable markers. The RUCAM scale has been proposed to identify causal relationship between offending drug and liver injury. Although, there is a need to validate a new and more accurate method of DILI identification, it is currently feasible to develop some refinements of the RUCAM scale in order to improve its capability.
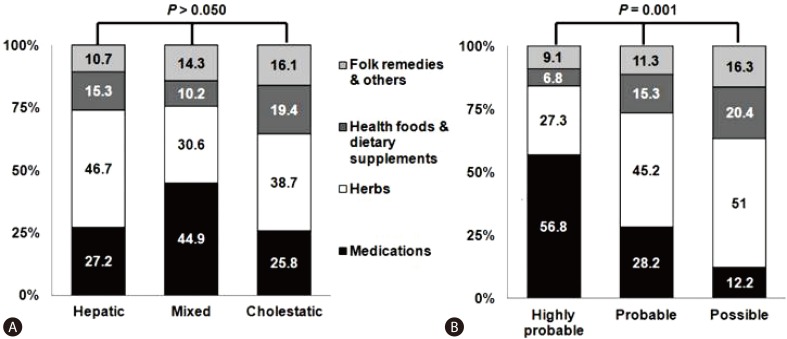
Suk et al8 suggested that the RUCAM score is found to be higher for medications than for other causes (Fig. 3). The main problem in applying the RUCAM score to 'herbs', 'health foods or dietary supplements', or 'folk remedies' is the time criteria. For instance, when the evidence of liver injury is identified ≥15 (hepatocellular type) and 30 days (mixed or cholestatic type) after the last day of ingestion, the drug is excluded from the study. Twenty one cases that were linked to 'herbs' did not meet the time to onset criteria of the RUCAM. Also, applications of the RUCAM score to the cases elicited by 'herbs', 'health foods or dietary supplements', or 'folk remedies' are hampered by the lack of previous information and incompatible time criteria. Therefore, a more objective and reproducible tool that could be used for causality assessment, especially for 'herbs', 'health foods or dietary supplements', or 'folk remedies', is needed.
Figure 3.
Classification of patients. (A) Types of drug induced liver injury by etiology. (B) Roussel Uclaf Causality Assessment Method score by etiology.8
DILI vigilance system
Currently, a nationwide PharmacoVigilance Research Network (PVNet) has been established as an adverse medication-reaction monitoring system in Korea. However, systems that specifically monitor for reaction to 'herbs', 'health foods or dietary supplements', or 'folk remedies' are not yet established. Many clinicians, including doctors of Oriental Medicine, are unaware of the diversity of potential danger of 'herbs', 'health foods or dietary supplements', or 'folk remedies'. As a result, it has been difficult to assess the possible sources of problems (for example, misuse or abuse, adulteration, or contamination of herbal medicine) that resulted in DILI. To collect and analyze the cases of DILI caused by 'herbs', 'health foods or dietary supplements', or 'folk remedies', a reporting system similar to the Drug-Induced Liver Injury Network (DILIN)7 in the United States should be established as soon as possible in Korea.
Abbreviations
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CAM
complementary and alternative medicines
- DDW-J
Digestive Disease Week-Japan
- DILI
drug-induced liver injury
- DILIN
Drug-Induced Liver Injury Network
- DLST
drug lymphocyte stimulation test
- RUCAM
Roussel Uclaf Causality Assessment Method
Footnotes
The authors have no conflicts to disclose.
References
- 1.Vuppalanchi R, Liangpunsakul S, Chalasani N. Etiology of new-onset jaundice: how often is it caused by idiosyncratic drug-induced liver injury in the United States? Am J Gastroenterol. 2007;102:558–562. doi: 10.1111/j.1572-0241.2006.01019.x. [DOI] [PubMed] [Google Scholar]
- 2.Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–2404. doi: 10.1038/ajg.2010.287. [DOI] [PubMed] [Google Scholar]
- 3.Fontana RJ, Watkins PB, Bonkovsky HL, Chalasani N, Davern T, Serrano J, et al. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32:55–64. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norris W, Paredes AH, Lewis JH. Drug-induced liver injury in 2007. Curr Opin Gastroenterol. 2008;24:287–297. doi: 10.1097/MOG.0b013e3282f9764b. [DOI] [PubMed] [Google Scholar]
- 5.Au JS, Navarro VJ, Rossi S. Review article: Drug-induced liver injury-its pathophysiology and evolving diagnostic tools. Aliment Pharmacol Ther. 2011;34:11–20. doi: 10.1111/j.1365-2036.2011.04674.x. [DOI] [PubMed] [Google Scholar]
- 6.Pugh AJ, Barve AJ, Falkner K, Patel M, McClain CJ. Drug-induced hepatotoxicity or drug-induced liver injury. Clin Liver Dis. 2009;13:277–294. doi: 10.1016/j.cld.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Watkins PB. Drug-induced liver injury network. Am J Gastroenterol. 2008;103:1574–1575. doi: 10.1111/j.1572-0241.2008.01880_8.x. [DOI] [PubMed] [Google Scholar]
- 8.Suk KT, Kim DJ, Kim CH, Park SH, Yoon JH, Kim YS, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012 Jun 26; doi: 10.1038/ajg.2012.138. doi: 10.1038/ajg.2012.138 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Kim DJ, Ahn BM, Choe SG, Shon JH, Seo JI, Park SH, et al. Preliminary multicenter study about toxic hepatitis in Korea. Korean J Hepatol. 2004;10(Suppl 2):80–86. [Google Scholar]
- 10.Invernizzi P. Drug-induced liver injury: is it time for genetics to change our clinical practice? J Hepatol. 2010;53:993–994. doi: 10.1016/j.jhep.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Daly AK. Drug-induced liver injury: past, present and future. Pharmacogenomics. 2010;11:607–611. doi: 10.2217/pgs.10.24. [DOI] [PubMed] [Google Scholar]
- 12.Bae SH, Kim DH, Bae YS, Lee KJ, Kim DW, Yoon JB, et al. Toxic hepatitis associated with Polygoni multiflori. Korean J Hepatol. 2010;16:182–186. doi: 10.3350/kjhep.2010.16.2.182. [DOI] [PubMed] [Google Scholar]
- 13.Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov. 2005;4:489–499. doi: 10.1038/nrd1750. [DOI] [PubMed] [Google Scholar]
- 14.Russmann S, Jetter A, Kullak-Ublick GA. Pharmacogenetics of drug-induced liver injury. Hepatology. 2010;52:748–761. doi: 10.1002/hep.23720. [DOI] [PubMed] [Google Scholar]
- 15.Russmann S, Kullak-Ublick GA, Grattagliano I. Current concepts of mechanisms in drug-induced hepatotoxicity. Curr Med Chem. 2009;16:3041–3053. doi: 10.2174/092986709788803097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aithal GP, Ramsay L, Daly AK, Sonchit N, Leathart JB, Alexander G, et al. Hepatic adducts, circulating antibodies, and cytokine polymorphisms in patients with diclofenac hepatotoxicity. Hepatology. 2004;39:1430–1440. doi: 10.1002/hep.20205. [DOI] [PubMed] [Google Scholar]
- 17.Lucena MI, Andrade RJ, Martínez C, Ulzurrun E, García-Martín E, Borraz Y, et al. Glutathione S-transferase m1 and t1 null genotypes increase susceptibility to idiosyncratic drug-induced liver injury. Hepatology. 2008;48:588–596. doi: 10.1002/hep.22370. [DOI] [PubMed] [Google Scholar]
- 18.Pachkoria K, Lucena MI, Crespo E, Ruiz-Cabello F, Lopez-Ortega S, Fernandez MA, et al. Analysis of IL-10, IL-4 and TNF-alpha polymorphisms in drug-induced liver injury (DILI) and its outcome. J Hepatol. 2008;49:107–114. doi: 10.1016/j.jhep.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Ong MM, Latchoumycandane C, Boelsterli UA. Troglitazone-induced hepatic necrosis in an animal model of silent genetic mitochondrial abnormalities. Toxicol Sci. 2007;97:205–213. doi: 10.1093/toxsci/kfl180. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 21.Kaplowitz N, Than TA, Shinohara M, Ji C. Endoplasmic reticulum stress and liver injury. Semin Liver Dis. 2007;27:367–377. doi: 10.1055/s-2007-991513. [DOI] [PubMed] [Google Scholar]
- 22.Hutchinson TA, Lane DA. Assessing methods for causality assessment of suspected adverse drug reactions. J Clin Epidemiol. 1989;42:5–16. doi: 10.1016/0895-4356(89)90020-6. [DOI] [PubMed] [Google Scholar]
- 23.Rochon J, Protiva P, Seeff LB, Fontana RJ, Liangpunsakul S, Watkins PB, et al. Reliability of the Roussel Uclaf Causality Assessment Method for assessing causality in drug-induced liver injury. Hepatology. 2008;48:1175–1183. doi: 10.1002/hep.22442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayashi PH. Causality assessment in drug-induced liver injury. Semin Liver Dis. 2009;29:348–356. doi: 10.1055/s-0029-1240003. [DOI] [PubMed] [Google Scholar]
- 25.Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs--II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol. 1993;46:1331–1336. doi: 10.1016/0895-4356(93)90102-7. [DOI] [PubMed] [Google Scholar]
- 26.Danan G, Benichou C. Causality assessment of adverse reactions to drugs-I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1330. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 27.Rockey DC, Seeff LB, Rochon J, Freston J, Chalasani N, Bonacini M, et al. Causality assessment in drug-induced liver injury using a structured expert opinion process: comparison to the Roussel-Uclaf causality assessment method. Hepatology. 2010;51:2117–2126. doi: 10.1002/hep.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maria VA, Victorino RM. Development and validation of a clinical scale for the diagnosis of drug-induced hepatitis. Hepatology. 1997;26:664–669. doi: 10.1002/hep.510260319. [DOI] [PubMed] [Google Scholar]
- 29.Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García-Ruiz E, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–521. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Lucena MI, Camargo R, Andrade RJ, Perez-Sanchez CJ, Sanchez De La Cuesta F. Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology. 2001;33:123–130. doi: 10.1053/jhep.2001.20645. [DOI] [PubMed] [Google Scholar]
- 31.Takikawa H, Takamori Y, Kumagi T, Onji M, Watanabe M, Shibuya A, et al. Assessment of 287 Japanese cases of drug induced liver injury by the diagnostic scale of the International Consensus Meeting. Hepatol Res. 2003;27:192–195. doi: 10.1016/s1386-6346(03)00232-8. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe M, Shibuya A. Validity study of a new diagnostic scale for drug-induced liver injury in Japan-comparison with two previous scales. Hepatol Res. 2004;30:148–154. doi: 10.1016/j.hepres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Watkins PB. Biomarkers for the diagnosis and management of drug-induced liver injury. Semin Liver Dis. 2009;29:393–399. doi: 10.1055/s-0029-1240008. [DOI] [PubMed] [Google Scholar]
- 34.Ari E, Yilmaz Y, Kedrah AE, Alahdab Y, Cakalagaoglu F, Arikan H, et al. Protective effect of the vasopressin agonist terlipressin in a rat model of contrast-induced nephropathy. Am J Nephrol. 2011;33:269–276. doi: 10.1159/000324764. [DOI] [PubMed] [Google Scholar]
- 35.Watkins PB, Seeff LB. Drug-induced liver injury: summary of a single topic clinical research conference. Hepatology. 2006;43:618–631. doi: 10.1002/hep.21095. [DOI] [PubMed] [Google Scholar]
- 36.Andrade RJ, Lucena MI, Kaplowitz N, García-Muņoz B, Borraz Y, Pachkoria K, et al. Outcome of acute idiosyncratic drug-induced liver injury: Long-term follow-up in a hepatotoxicity registry. Hepatology. 2006;44:1581–1588. doi: 10.1002/hep.21424. [DOI] [PubMed] [Google Scholar]
- 37.Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 1995;333:1118–1127. doi: 10.1056/NEJM199510263331706. [DOI] [PubMed] [Google Scholar]
- 38.Zimmerman H. Hepatotoxicity the adverse effects of drugs and other chemicals on the liver. Philadelphia: Lippincott, Williams & Wilkins; 1999. [Google Scholar]
- 39.Reuben A. Hy's law. Hepatology. 2004;39:574–578. doi: 10.1002/hep.20081. [DOI] [PubMed] [Google Scholar]
- 40.Temple R. Hy's law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf. 2006;15:241–243. doi: 10.1002/pds.1211. [DOI] [PubMed] [Google Scholar]
- 41.Björnsson E. Drug-induced liver injury: Hy's rule revisited. Clin Pharmacol Ther. 2006;79:521–528. doi: 10.1016/j.clpt.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Björnsson E, Olsson R. Suspected drug-induced liver fatalities reported to the WHO database. Dig Liver Dis. 2006;38:33–38. doi: 10.1016/j.dld.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 43.De Valle MB, Av Klinteberg V, Alem N, Olsson R, Björnsson E. Drug-induced liver injury in a Swedish University hospital out-patient hepatology clinic. Aliment Pharmacol Ther. 2006;24:1187–1195. doi: 10.1111/j.1365-2036.2006.03117.x. [DOI] [PubMed] [Google Scholar]
- 44.Tan HH, Chang CY, Martin P. Acetaminophen hepatotoxicity: current management. Mt Sinai J Med. 2009;76:75–83. doi: 10.1002/msj.20065. [DOI] [PubMed] [Google Scholar]
- 45.Björnsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. 2005;42:481–489. doi: 10.1002/hep.20800. [DOI] [PubMed] [Google Scholar]
- 46.Reuben A, Koch DG, Lee WM Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–2076. doi: 10.1002/hep.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 48.Wei G, Bergquist A, Broomé U, Lindgren S, Wallerstedt S, Almer S, et al. Acute liver failure in Sweden: etiology and outcome. J Intern Med. 2007;262:393–401. doi: 10.1111/j.1365-2796.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- 49.Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856–864. 864.e1. doi: 10.1053/j.gastro.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt LE, Larsen FS. MELD score as a predictor of liver failure and death in patients with acetaminophen-induced liver injury. Hepatology. 2007;45:789–796. doi: 10.1002/hep.21503. [DOI] [PubMed] [Google Scholar]
- 51.Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–1934. 1934.e1–1934.e4. doi: 10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Björnsson E. Review article: drug-induced liver injury in clinical practice. Aliment Pharmacol Ther. 2010;32:3–13. doi: 10.1111/j.1365-2036.2010.04320.x. [DOI] [PubMed] [Google Scholar]
- 53.Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–1934. 1934.e1–1934.e4. doi: 10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]