Abstract
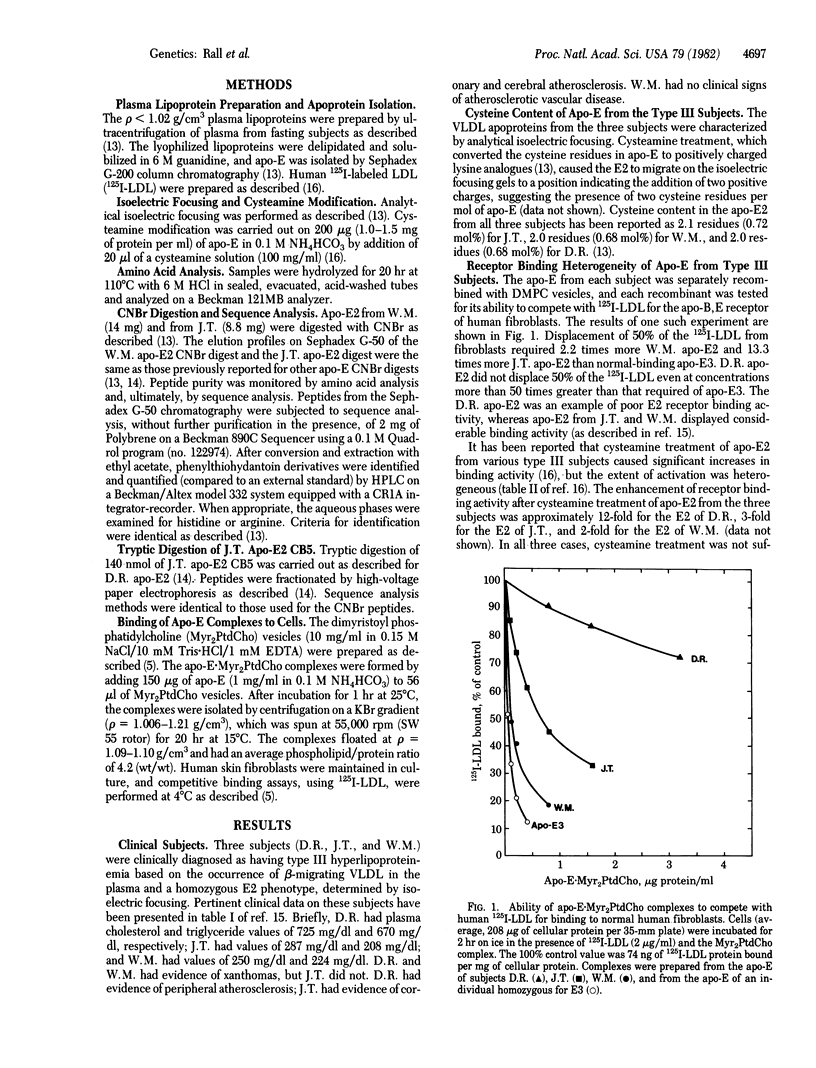
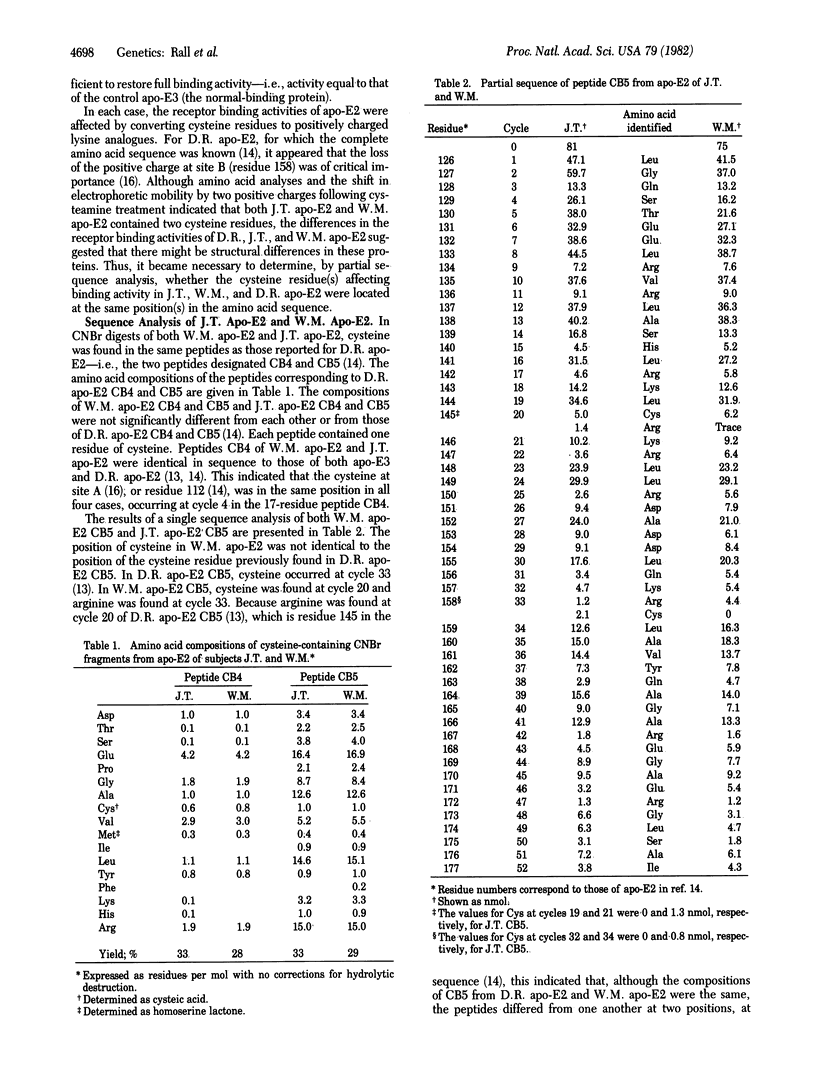
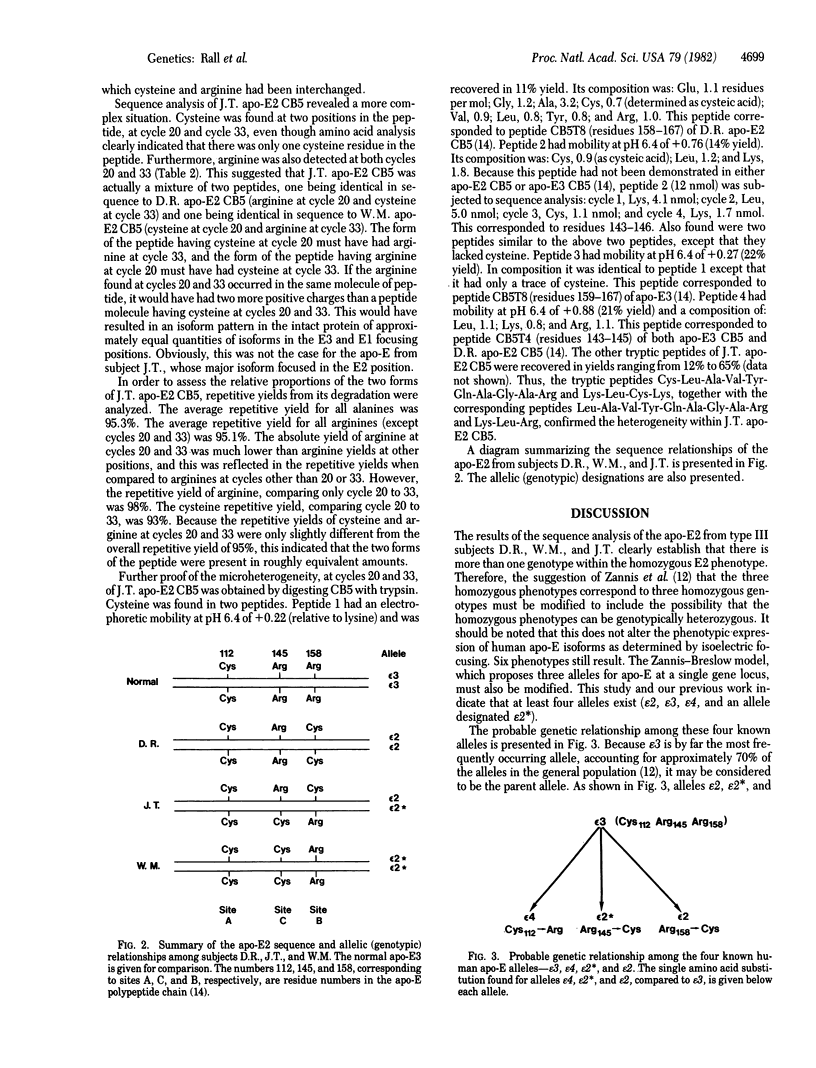
The three major isoforms of human apolipoprotein E (apo-E2, -E3, and -E4) are coded for by three alleles (epsilon 2, epsilon 3, and epsilon 4) which have a common genetic locus. Previously, we demonstrated that E2, E3, and E4 differ in primary structure from one another at two substitution sites, site A (residue 112) and site B (residue 158). At sites A/B, apo-E2, -E3, and -E4 contain cysteine/cysteine, cysteine/arginine, and arginine/arginine, respectively. We demonstrated that the substitution of cysteine for arginine at site B is at least partly responsible for the defective binding of apo-E2 to human fibroblast low density lipoprotein receptors, compared to the normal binding activity of apo-E3 or -E4. Subjects with the genetic disorder type III hyperlipoproteinemia are phenotypically homozygous for apo-E2, but the binding activity of apo-E to the fibroblast receptor differs considerably from one type III individual to another. We therefore undertook a partial comparative sequence analysis of apo-E2 from three type III subjects whose apo-E displayed this heterogeneity. The subject with the poorest binding apo-E2 was genotypically homozygous for an apo-E allele (epsilon 2); cysteine was found at sites A and B. The subject with the most active apo-E2 was genotypically homozygous for an apo-E allele (epsilon 2); cystine was found at site A and at a new site (site C, residue 145). The epsilon 2 allele specifies a protein that has arginine at site B (residue 158); the epsilon 2 allele specifies a protein that has arginine at site C (residue 145). Therefore, the two alleles differ from one another by cysteine/arginine interchanges at two positions, sites B and C. The third subject, whose apo-E2 displayed binding activity intermediate between the activities of the other two, was genotypically heterozygous, having one epsilon 2 allele and one epsilon 2 allele. The intermediate binding activity of apo-E2 from this subject resulted from having a mixture of severely defective apo-E (specified by epsilon 2) and slightly defective apo-E (specified by epsilon 2).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Havel R. J., Chao Y., Windler E. E., Kotite L., Guo L. S. Isoprotein specificity in the hepatic uptake of apolipoprotein E and the pathogenesis of familial dysbetalipoproteinemia. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4349–4353. doi: 10.1073/pnas.77.7.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D. Y., Innerarity T. L., Mahley R. W. Lipoprotein binding to canine hepatic membranes. Metabolically distinct apo-E and apo-B,E receptors. J Biol Chem. 1981 Jun 10;256(11):5646–5655. [PubMed] [Google Scholar]
- Innerarity T. L., Mahley R. W. Enhanced binding by cultured human fibroblasts of apo-E-containing lipoproteins as compared with low density lipoproteins. Biochemistry. 1978 Apr 18;17(8):1440–1447. doi: 10.1021/bi00601a013. [DOI] [PubMed] [Google Scholar]
- Innerarity T. L., Pitas R. E., Mahley R. W. Binding of arginine-rich (E) apoprotein after recombination with phospholipid vesicles to the low density lipoprotein receptors of fibroblasts. J Biol Chem. 1979 May 25;254(10):4186–4190. [PubMed] [Google Scholar]
- Mahley R. W., Hui D. Y., Innerarity T. L., Weisgraber K. H. Two independent lipoprotein receptors on hepatic membranes of dog, swine, and man. Apo-B,E and apo-E receptors. J Clin Invest. 1981 Nov;68(5):1197–1206. doi: 10.1172/JCI110365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnan A., Havel R. J., Kane J. P., Kotite L. Characterization of human very low density lipoproteins containing two electrophoretic populations: double pre-beta lipoproteinemia and primary dysbetalipoproteinemia. J Lipid Res. 1977 Sep;18(5):613–622. [PubMed] [Google Scholar]
- Rall S. C., Jr, Weisgraber K. H., Mahley R. W. Human apolipoprotein E. The complete amino acid sequence. J Biol Chem. 1982 Apr 25;257(8):4171–4178. [PubMed] [Google Scholar]
- Schneider W. J., Kovanen P. T., Brown M. S., Goldstein J. L., Utermann G., Weber W., Havel R. J., Kotite L., Kane J. P., Innerarity T. L. Familial dysbetalipoproteinemia. Abnormal binding of mutant apoprotein E to low density lipoprotein receptors of human fibroblasts and membranes from liver and adrenal of rats, rabbits, and cows. J Clin Invest. 1981 Oct;68(4):1075–1085. doi: 10.1172/JCI110330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill B. C., Innerarity T. L., Mahley R. W. Rapid hepatic clearance of the canine lipoproteins containing only the E apoprotein by a high affinity receptor. Identity with the chylomicron remnant transport process. J Biol Chem. 1980 Mar 10;255(5):1804–1807. [PubMed] [Google Scholar]
- Utermann G., Jaeschke M., Menzel J. Familial hyperlipoproteinemia type III: deficiency of a specific apolipoprotein (apo E-III) in the very-low-density lipoproteins. FEBS Lett. 1975 Aug 15;56(2):352–355. doi: 10.1016/0014-5793(75)81125-2. [DOI] [PubMed] [Google Scholar]
- Utermann G., Vogelberg K. H., Steinmetz A., Schoenborn W., Pruin N., Jaeschke M., Hees M., Canzler H. Polymorphism of apolipoprotein E. II. Genetics of hyperlipoproteinemia type III. Clin Genet. 1979 Jan;15(1):37–62. [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Mahley R. W. Abnormal lipoprotein receptor-binding activity of the human E apoprotein due to cysteine-arginine interchange at a single site. J Biol Chem. 1982 Mar 10;257(5):2518–2521. [PubMed] [Google Scholar]
- Weisgraber K. H., Rall S. C., Jr, Mahley R. W. Human E apoprotein heterogeneity. Cysteine-arginine interchanges in the amino acid sequence of the apo-E isoforms. J Biol Chem. 1981 Sep 10;256(17):9077–9083. [PubMed] [Google Scholar]
- Zannis V. I., Breslow J. L. Characterization of a unique human apolipoprotein E variant associated with type III hyperlipoproteinemia. J Biol Chem. 1980 Mar 10;255(5):1759–1762. [PubMed] [Google Scholar]
- Zannis V. I., Breslow J. L. Human very low density lipoprotein apolipoprotein E isoprotein polymorphism is explained by genetic variation and posttranslational modification. Biochemistry. 1981 Feb 17;20(4):1033–1041. doi: 10.1021/bi00507a059. [DOI] [PubMed] [Google Scholar]
- Zannis V. I., Just P. W., Breslow J. L. Human apolipoprotein E isoprotein subclasses are genetically determined. Am J Hum Genet. 1981 Jan;33(1):11–24. [PMC free article] [PubMed] [Google Scholar]



