Abstract
Swimming fish leave wakes containing hydrodynamic and chemical traces. These traces mark their swim paths and could guide predators. We now show that nocturnal European catfish (Silurus glanis) locate a piscine prey (guppy, Poecilia reticulata) by accurately tracking its three-dimensional swim path before an attack in the absence of visible light. Wakes that were up to 10 s old were followed over distances up to 55 prey-body lengths in our setup. These results demonstrate that prey wakes remain sufficiently identifiable to guide predators, and to extend considerably the area in which prey is detectable. Moreover, wakes elicit rear attacks, which may be more difficult to detect by prey. Wake tracking may be a common strategy among aquatic predators.
When an animal swims, chemical and hydrodynamic stimuli persist in its wake for some time after it has left the vicinity (1, 2). Previous studies on predatory fish using olfactory or mechanosensory cues to localize moving prey are few in number and indirect (3, 4). Most studies on predator–prey interactions in fish have used predominantly visual predators under well lit conditions (5). The niches of many piscivorous fish, however, require them to hunt at night or at depths where the limited penetration of solar, sidereal, or lunar illumination limits the utility of visual senses (6). We hypothesized that in these circumstances, wakes left by prey fish are used by predatory fish to detect and track their prey in three dimensions, analogous to the way in which dogs or snakes follow the two-dimensional tracks left by their terrestrial prey (7, 8). To test this hypothesis, we analyzed the predatory strategy of a nocturnal catfish (Silurus glanis) as it found and attacked swimming prey fish (guppies, Poecilia reticulata). The use of a prey's wake can be distinguished from visual, acoustical, and electrical tracking of prey by path analysis. In all but wake tracking, the predator perceives the instantaneous location of its prey and will approach it directly or in an arc, intercepting the prey's path (9, 10). An indication, therefore, of wake tracking is the similarity of the paths of prey and predator through space with a significant time lag.
Materials and Methods
We used a video-based infrared-illuminated system, maintaining both prey and predator in visual blackout conditions and allowing us to make three-dimensional evaluations of their swim paths. This system consisted of a glass test aquarium (120 cm × 60 cm, filled to a height of 40 cm) illuminated by infrared back lighting. The infrared used was in the 810–950-nm range (maximum at 870 nm), which is outside the range of absorption of the visual pigments of fish (11). Catfish conditioned to react to visual stimuli do not react under infrared illumination, confirming that they cannot see in infrared (K. Pohlmann, personal observation). Fish behavior was recorded on digital video by using two IR-sensitive cameras from different directions. The two recordings were synchronized accurately to the frame. Guppies (total lengths 2.0–5.1 cm) were chosen as prey for their slow and clumsy swimming behavior and for their low tendency to swim or rest close to walls. Guppies use undulatory and push-and-coast swimming. The wakes caused by both of these swimming modes are well described (2, 12, 13). Four different catfish were used as predators, total lengths 20–25 cm. They were accustomed to feeding on live piscine prey.
Each trial started after the catfish had been acclimated in the experimental tank for at least 1 h in darkness. The experimental room was entered through a double curtain to ensure total darkness and one individual guppy was added with a small amount of water (<50 ml) into the middle of the experimental tank. Five min after the prey had been consumed (viewed on monitors next door), the next prey was added. A trial ended when 10 prey fish had been consumed or was aborted when the added prey fish were not consumed within 20 min.
Attack Characterization.
All sequences leading to attacks of the predator on a prey were analyzed carefully from the two video recordings. All captures and snapping movements of a catfish directed at a guppy less than 1.5 cm away were considered attacks. The direction from which the guppy was attacked (top, below, sides, front, behind) was determined for each sequence. The direction of attacks did not change with the number of prior attacks (nominal logistic regression, P = 0.45). Nominal logistic regression is used to determine the effect of one or more predictors on a nominal (i.e., not quantitative) dependent variable. There were no significant effects of guppy gender or its total length, or of catfish identity on the direction of attacks (tested simultaneously by nominal logistic regression, P > 0.38). Therefore, all data were pooled for subsequent analyses.
We classified all attacks into three categories: (i) path-following (the predator swam along the same path as the prey, eventually attacking it); (ii) head-on encounters (the predator encountered the moving prey without prior path overlaps); and (iii) attacks on stationary guppies.
Quantitative Path Analyses.
To determine whether catfish were actually following the wakes of their prey, we digitized (at 25 Hz; Adobe premiere 5.1, Adobe Systems, Mountain View, CA) sequences with attacks occurring away from the walls to avoid path convergence resulting from both fish swimming along the wall. We chose 22 attacks classified as path-following; for the two other types, all attacks away from the walls were digitized. The digitized sequences ended with the attack or capture and started 2 s before the predator seemed to respond to the presence of the prey. To digitize swimming paths, we tracked the positions of the tips of the heads of both predator and prey by using motion analysis software (winanalyze 1.1, Weinberger, Karlsruhe, Germany).
The resulting three-dimensional swim paths of predator and prey were smoothed by a running average over 10 points to eliminate predator head movement and tracking inaccuracies. Then, these digitized swim paths were plotted (for examples see Figs. 1 and 2). To establish a quantitative comparison of swim paths independent of the swimming velocities of predator or prey, new points were set at regular intervals of 2 mm on each path. To determine whether the predator had been swimming along the path of the prey, the swimming direction in each point of the predator's path was subtracted from the direction of the closest point of the prey's path that occurred simultaneously or prior.
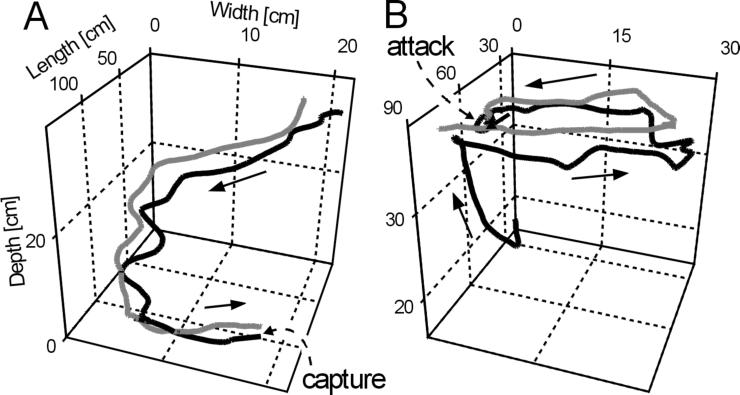
Figure 1.
Examples of smoothed three-dimensional swim paths of predator and prey prior to attacks classified as wake following. Black, predator; gray, prey. The numbers depict cm and correspond to calibrated positions in the test tank. Arrows indicate the swimming direction. The three planes drawn do not depict the walls of the tank: the bottom plane was at a depth = 0 cm, the surface plane was at a depth = 40 cm, and the walls were at length = 0 and 120 with widths = −5 and 55 cm. Note different x, y, and z scales.
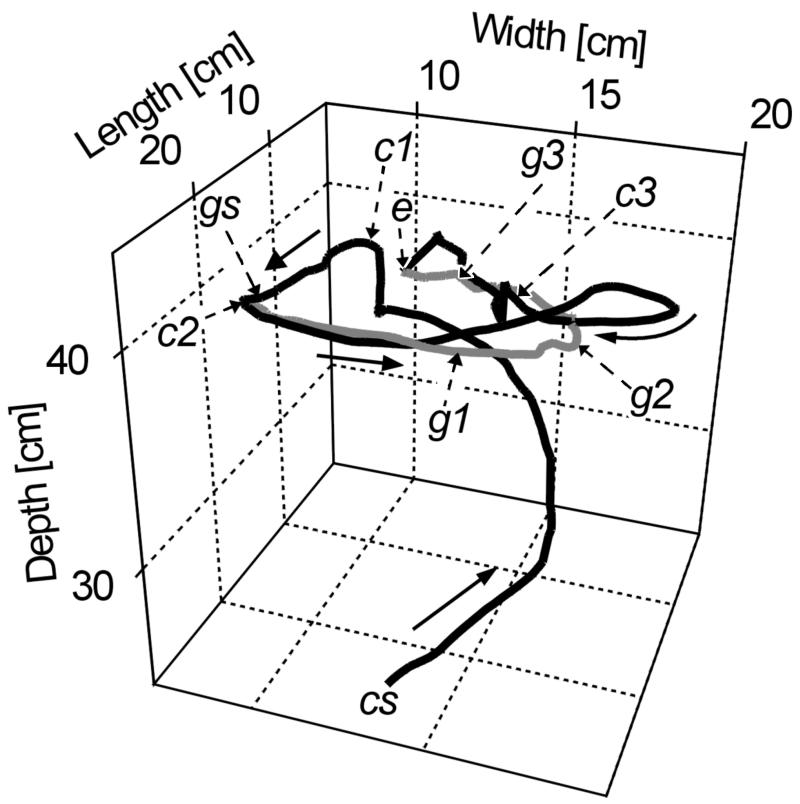
Figure 2.
Three-dimensional plot with temporal information of an attack categorized as wake following. Black, predator; gray, prey. The numbers depict cm and correspond to calibrated positions in the test tank. Solid arrows indicate the swimming direction. Three points in time (1 = 11.3 s, 2 = 8.6 s, and 3 = 3.4 s before the attack) were chosen to indicate the locations of both fish: c1–c3 correspond to positions of the catfish and g1–g3 correspond to those of the guppy (e.g., c2 and g2 are synchronous positions). Also, the first (cs and gs) and last (e) points of both paths are at the same time. The path-following appears to begin when the prey is at g2 and when the predator at c2. Note different x, y, and z scales.
Indices of path similarity were computed from the distribution of these differences in swimming direction for each pair of swim paths. As indices, we used the medians of the distributions to express central tendency and quartile differences (75–25% quartile) to express the spread of the distributions. Separate indices were computed for the medians and quartile differences in the orthogonal xy and xz planes for each pair of swim paths. Fish swimming along the same path, regardless of how complex or convoluted, should have small differences (medians around 0 and small quartile differences) in swimming directions, whereas for fish swimming in independent paths, absolute values of these parameters should be considerably larger.
Nearest neighborhood discriminant analysis (k = 3) was used to determine the reliability of our three predetermined attack categories. This analysis was calculated on the basis of medians and quartile differences of distances between nearest points and differences in swimming directions. The proportion of misclassification was estimated by using the cross-validation approach, which is suitable for low and unequal sample sizes (14, 15).
All calculations, and the discriminant and cross-validation analyses (PROC DISCRIM, METHOD = NPAR) were done by using SAS 8; nominal logistic regressions and χ2 tests for the goodness of fit were performed in JMP 3.15.
Results
Of the 94 observed attacks, 59 resulted in successful captures of guppies. Seventy-five (80%) of all attacks occurred on moving prey, which is significantly higher than expected from the proportion of time guppies spent swimming (43% of total time, averaged from 8 arbitrarily chosen sequences of 31-min total duration; χ2 test for goodness of fit; P < 0.0001).
Most attacks were initiated from behind the prey fish (46% compared with 21% from the front, 9% from the two sides, 6% from above, and 18% from below). This proportion is significantly higher than expected if predators had no preferred attack direction (16.7%, χ2 test for goodness of fit; P < 0.0001). Of all rear attacks, 95% were directed toward moving prey.
Of the observed 94 attacks, 57 were categorized as path-following, 23 were categorized as head-on encounters, and 14 were categorized as attacks on stationary guppies. The categorization was confirmed graphically by plotting all digitized swim paths in three dimensions. Fig. 1 A and B shows examples of smoothed swim paths of predator and prey before attacks categorized as path-following. Fig. 2 depicts the spatiotemporal relations between predator and prey in another example categorized as path-following. The positions are indicated at 5 points in time: start, end, and 1–3 (catfish, c; guppy, g; Fig. 2).
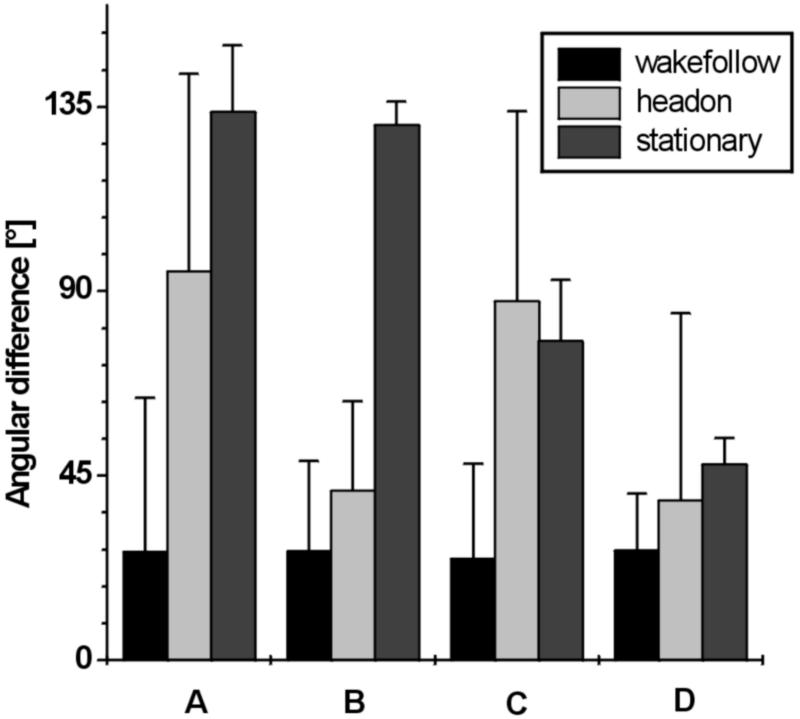
Indices of path similarity computed from the distribution of the differences in swimming direction supported our classification: The medians and spread of the differences in swimming direction of attacks classified as path-following were small and much lower than those of the head-on encounters and attacks on stationary guppies (Fig. 3). Thus, in sequences categorized as wake-following, predator and prey were predominantly swimming in the same direction when, with a delay, they occupied the same location.
Figure 3.
Means and standard deviations of differences in swimming direction of predator and prey before attacks, comparing three attack categories: path-following, head-on encounters, and attacks on stationary guppies. A, medians in xy plane. B, quartile differences in xy plane. C, medians in xz plane. D, quartile differences in xz plane.
Nearest neighborhood discriminant analysis and crossvalidation further confirmed our categorization on the basis of quantitative criteria. Of the 34 digitized swim paths (22 path-following, 7 head-on encounters, 5 attacks on stationary guppies), only the two shortest were misclassified: one path-following was classified as a head-on encounter, and one head-on encounter was classified as an attack on a stationary guppy.
These results confirm that in the majority of all attacks, catfish were swimming along the same path as their prey before the attack.
The digitized sequences showed that catfish followed the wake of their prey for up to 121 cm (∼55 prey-body lengths) and for as long as 33 s with maximal distances of 40 cm between animals despite the confined space of our aquarium. The median length of tracked-guppy paths was 47 cm. The path of the prey was up to 10.3 s old when encountered and subsequently followed by the predator. Distances between the prey and predator ranged from 40 to 6 cm (median 12 cm) at the onset of tracking. The median distance during all digitized tracking sequences was 7.6 cm with distances gradually decreasing as the predator approached the prey (see Fig. 2).
Discussion
That the proportion of fish being attacked when moving is significantly larger than the proportion of time guppies spend moving indicates that catfish find moving guppies easier to detect and localize. This finding could imply wake detection but also the use of vision, hearing, and other senses that detect the instantaneous position of moving prey. However, we discovered here that moving fish were attacked predominantly from behind, that distances between fish at the onset and during path-following were several prey lengths, and that the majority of attacks occurred after path-following. These results make it most likely that the predator followed chemical/hydrodynamic cues in the wake of the prey. Chemical cues in the wake could be detected by olfaction or the extensive sense of taste; the latter is used by similar catfish in localizing nonmoving food (26). Hydrodynamic cues could be detected by the lateral line.
As alternatives to using hydrodynamic and/or chemical cues marking “past” prey positions in the wake, the predator could have used visual, acoustic, or electric cues radiating directly from the swimming prey, revealing its “instantaneous” position. Predators perceiving the instantaneous position of a prey would not follow more or less convoluted trails, i.e., past positions, over several prey-body lengths if they knew the actual prey location and could make a direct attack. Predatory strategies other than wake tracking would thus result in different swim paths or different spatiotemporal relations between predator and prey (as further explained below). Catfish are known to have a keen sense of hearing (16) and passive electroreception (17, 18). These senses as well as vision could be used to determine the instantaneous prey position.
Catfish do not seem to use their eyes for food detection (K. Pohlmann, personal observation, and J. Atema, personal communication) and, in addition, we did our experiments under infrared illumination eliminating visual cues in the spectrum generally perceivable by fish. Observations made when maintaining catfish in the lab indicate that catfish in general do not orient toward the visual image of food even in visible light. Acoustically guided attacks toward a target that is emitting sound continuously or in pulses (i.e., with every tail beat) are expected to come from any side but preferably not from behind the prey. Acoustic stimuli have been presumed to occur during swimming (19). In other experiments, we introduced a highly sensitive hydrophone (Type 8101; Brüel & Kjaer, Norcross, GA) into the tank. However, we never succeeded in recording any sounds from small fish like those used in the present experiment. In addition, in our experimental aquarium the background noise originating from pumps and cooling systems was so high (90 dB relative to 1 μPa, 0–200 Hz) that they would have masked subtle acoustic stimuli. Therefore, we conclude that neither visual nor acoustic stimuli were used to guide the wake following of the catfish.
Fish are surrounded by a dipole-like electric field that is detectable by catfish at about one prey-body length (20). When swimming, they also generate local hydrodynamic cues distinct from the wake [i.e., dipole-like flow fields (20)]. Neither of these fields is strongest behind the prey.
Swim paths of prey and predator would be similar also, at least over short distances, if the predator continuously sensed the instantaneous location of the moving prey and followed behind cautiously and closely, or if the prey sensed the nearby predator and swam away with the predator again following immediately. The prey, on sensing the predator, would be expected to react with escape movements. Rapid escape movements of the prey before an actual attack never occurred.
At greater distances (i.e., a few prey lengths) between predator and prey the predator should cut corners, resulting in more rapid turns; instead, we observed gradual curves along the trail of the prey.
Finally, the distance between predator and prey along their paths gives important clues to the sensory information most likely used by the predator. As depicted in Fig. 2, at time 1 (c1, g1) the distance between predator (c1) and prey (g1) is smaller (distance = 6 cm) than at time 2 (c2, g2, distance = 10 cm). If the catfish used any form of instantaneous position detection (vision, audition, or the local electric or hydrodynamic stimulus fields) and not the wake, the predator should turn toward the prey at time 1 and not continue straight until hitting the previous path of the prey.
Therefore, the high proportion of observed rear attacks and the similarities in swim paths when fish were still several prey lengths apart cannot be explained by random encounters or by visual, electrosensory, or acoustic orientation. Wake tracking is the most parsimonious explanation for the observed predatory behavior.
Tank constraints may have limited wake detection. Flow visualization using small particles revealed that the disturbances created by the swimming catfish overpowered the smaller wakes of the prey, thereby limiting the possible tracking distance. The small test aquarium increased the probability of random encounters and caused wake reflections off the walls. Despite these constraints, wake following was the most frequent occurrence preceding an attack.
Hanke et al. (2) showed that in still water, a wake can be measured by particle image velocimetry over a 3-min period, and concluded that fish should be able to derive directional information from a 60-s-old wake. We expect that the distance and duration over which a wake can be detected under quiet natural conditions with modest background flow are higher than the 10 s found in our experiment, with fewer reflections and predator-caused perturbations than in our tank.
In the wake of a swimming fish, there is hydrodynamic and chemical information (21). The hydrodynamic stimuli caused by fish swimming in different modes have been studied in detail (12, 13, 22, 23). The hydrodynamic structure of a wake may hold information on direction, swimming mode, and size of the fish (2, 22). Because there are characteristic changes during the aging of hydrodynamic structures (2), it should be possible for a fish to estimate how long ago another fish had been there. The lateral line is sensitive enough to detect these stimuli and filters the relevant details (24, 25).
Chemical signals contained in the wake provide information about identity, and possibly distance and direction, of the prey. Size can be assessed from the expansion. Distance and direction are coded in steepness of the chemical gradients in dispersing odor patches (21). In catfish, chemical (taste) receptors are present in high densities on the whole body surface (26). For catfish, gustation is the major sense involved in locating and ingesting nonmoving food (26), and they exhibit true gradient searches to locate nonmoving food items in stagnant water (27). Direction and age of the odor trail can be assessed by instantaneous chemical comparison using bilateral receptors (e.g., on the barbels) or temporal (sequential) comparison with only one sensory organ (21). Our catfish showed an enhanced interest in places where the prey had spent extended time during previous intervals. It is possible that in these regions, chemical stimuli had accumulated. It is not known whether catfish use olfaction or taste for wake tracking. Future ablation experiments will reveal whether smell, taste, and/or hydrodynamic stimuli provide the sensory cues used during wake tracking.
Wakes are a ubiquitous consequence of physical objects moving through a fluid. Doall et al. (28) showed that copepods follow their mating partners by using chemical cues in the wakes. Our study shows that at least one species of teleost can make use of the hydrodynamic/chemical cues in the wake to track its prey. We suspect that exploitation of these cues is common among large animals that track moving prey through water.
Denhardt et al.¶ showed that harbor seals can be trained to follow the hydrodynamic trail produced by a propeller-driven minisubmarine, using their whiskers for sensing the water movements. The wakes of fish schools condition a much larger area than that of individual fish and provide even more conspicuous tracks to be used by predators (29). The advantages of wake tracking may have promoted special adaptations in both hydrodynamic- and olfactory-receptor systems. One might expect specialization in the lateral line system, for analyzing hydrodynamic details of the wakes (25), or chemosensory specialization, comparable to the forked tongues of snakes, that facilitates tropotactic tracking of prey trails (30). These specializations await further exploration.
Acknowledgments
We thank Drs. G. Gerlach, J. Atema, W. Hanke, and H.-P. Grossart as well as three anonymous reviewers for helpful comments on earlier versions of this manuscript; M. Schmid for help in digitizing swim paths; and W. Nagl for assistance in developing the statistical approach. O. Lübker provided the catfish. This study was supported by the German Research Foundation (SFB 454-C3) and Hoch-Schulbau-Förderungsgesetz. The protocol was approved by the German Ethics Commission, Regierungspräsidium Freiburg (G-97/56).
Footnotes
Dehnhardt, G., Hanke, W. & Mauck, B. (2000) Zool. Suppl. III 103, 16 (abstr.).
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Dusenbery D B. Sensory Ecology: How Organisms Acquire and Respond to Information. New York: Freeman; 1992. [Google Scholar]
- 2.Hanke W, Brücker C, Bleckmann H. J Exp Biol. 2000;203:1193–1200. doi: 10.1242/jeb.203.7.1193. [DOI] [PubMed] [Google Scholar]
- 3.Enger P S, Kalmijn A J, Sand O. In: The Mechanosensory Lateral Line: Neurobiology and Evolution. Coombs S, Görner P, Münz H, editors. New York: Springer; 1989. pp. 574–587. [Google Scholar]
- 4.Montgomery J C, Hamilton A R. Mar Freshwater Behav Physiol. 1992;30:209–223. [Google Scholar]
- 5.Gerking S D. Feeding Ecology of Fish. San Diego: Academic; 1994. [Google Scholar]
- 6.Helfman G S. In: Behaviour of Teleost Fishes. Pitcher T J, editor. London: Chapman & Hall; 1993. pp. 479–512. [Google Scholar]
- 7.Thesen A, Steen J B, Døving K B. J Exp Biol. 1993;180:247–251. doi: 10.1242/jeb.180.1.247. [DOI] [PubMed] [Google Scholar]
- 8.Webb J K, Shine R. Anim Behav. 1992;43:941–948. [Google Scholar]
- 9.Ohlberg R M, Worthington A H, Venator K R. J Comp Physiol A. 2000;186:155–162. doi: 10.1007/s003590050015. [DOI] [PubMed] [Google Scholar]
- 10.Kalmijn A J. In: Sensory Biology of Aquatic Animals. Atema J, Popper A N, Fay R R, Tavolga W N, editors. Berlin: Springer; 1988. pp. 151–186. [Google Scholar]
- 11.Dartnall H J A. In: Vision in Fishes—New Approaches in Research. Ali M A, editor. New York: Plenum; 1975. pp. 543–563. [Google Scholar]
- 12.McCutchen C W. In: Scale Effects in Locomotion. Pedley T J, editor. London: Academic; 1977. pp. 339–363. [Google Scholar]
- 13.Müller U K, Stamhuis E J, Videler J J. J Exp Biol. 2000;203:193–206. doi: 10.1242/jeb.203.2.193. [DOI] [PubMed] [Google Scholar]
- 14.Lachenbruch P A, Mickey L A. Technometrics. 1968;10:1–10. [Google Scholar]
- 15.SAS. SAS Procedures Guide. Vol. 1. Cary, NC: SAS Institute; 1993. p. 685. [Google Scholar]
- 16.Fay R R. Hearing In Vertebrates: A Psychophysics Databook. Winnetka, IL: Hill-Fay; 1988. [Google Scholar]
- 17.Peters R C, Wijland v F. J Comp Physiol. 1974;92:273–280. [Google Scholar]
- 18.Finger T E. In: Electroreception. Bullock T H, Heiligenberg W, editors. New York: Wiley; 1986. pp. 287–317. [Google Scholar]
- 19.Hawkins A D, Myrberg A A J. In: Bioacoustics, A Comparative Approach. Lewis B, editor. New York: Academic; 1983. pp. 347–405. [Google Scholar]
- 20.Kalmijn A J. Sensory Biology of Aquatic Animals. Berlin: Springer; 1988. pp. 83–129. [Google Scholar]
- 21.Atema J. Biol Bull. 1996;191:129–138. doi: 10.2307/1543074. [DOI] [PubMed] [Google Scholar]
- 22.Bleckmann H, Breithaupt T, Blickhan R, Tautz J. J Comp Physiol A. 1991;168:749–757. doi: 10.1007/BF00224363. [DOI] [PubMed] [Google Scholar]
- 23.Blickhan R, Krick C, Zehren D, Nachtigall W, Breithaupt T. Naturwissenschaften. 1992;79:220–221. [Google Scholar]
- 24.Bleckmann H. Reception of Hydrodynamic Stimuli in Aquatic and Semiaquatic Animals. Stuttgart: Gustav Fischer; 1994. [Google Scholar]
- 25.Engelmann J, Hanke W, Mogdans J, Bleckmann H. Nature (London) 2000;408:51–52. doi: 10.1038/35040706. [DOI] [PubMed] [Google Scholar]
- 26.Atema J. Brain Behav Evol. 1971;4:273–294. doi: 10.1159/000125438. [DOI] [PubMed] [Google Scholar]
- 27.Bardach J E, Todd J H, Crickmer R. Science. 1967;155:1276–1278. doi: 10.1126/science.155.3767.1276. [DOI] [PubMed] [Google Scholar]
- 28.Doall M H, Weissburg M J, Yen J. Philos Trans R Soc London B. 1998;353:681–689. doi: 10.1098/rstb.1998.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pitcher T J, Parrish J K. In: Behaviour of Teleost Fishes. Pitcher T J, editor. London: Chapman & Hall; 1993. pp. 363–439. [Google Scholar]
- 30.Schwenk K. Science. 1994;263:1573–1577. doi: 10.1126/science.263.5153.1573. [DOI] [PubMed] [Google Scholar]