Abstract
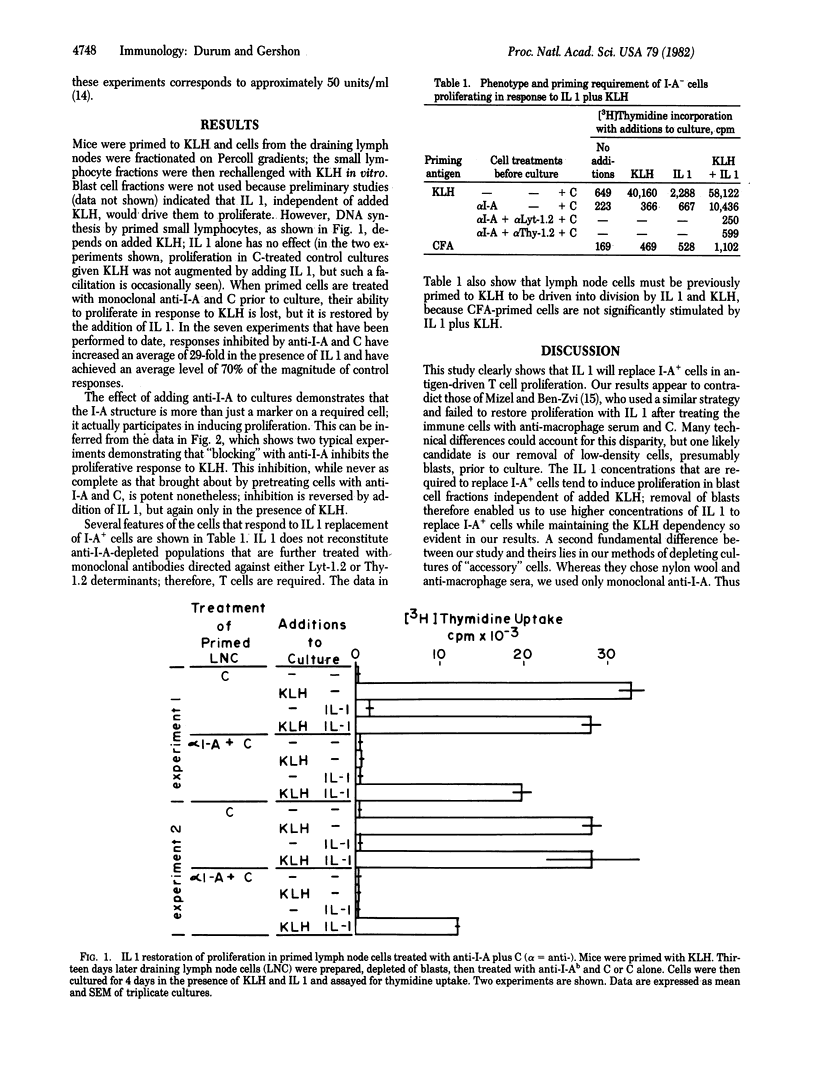
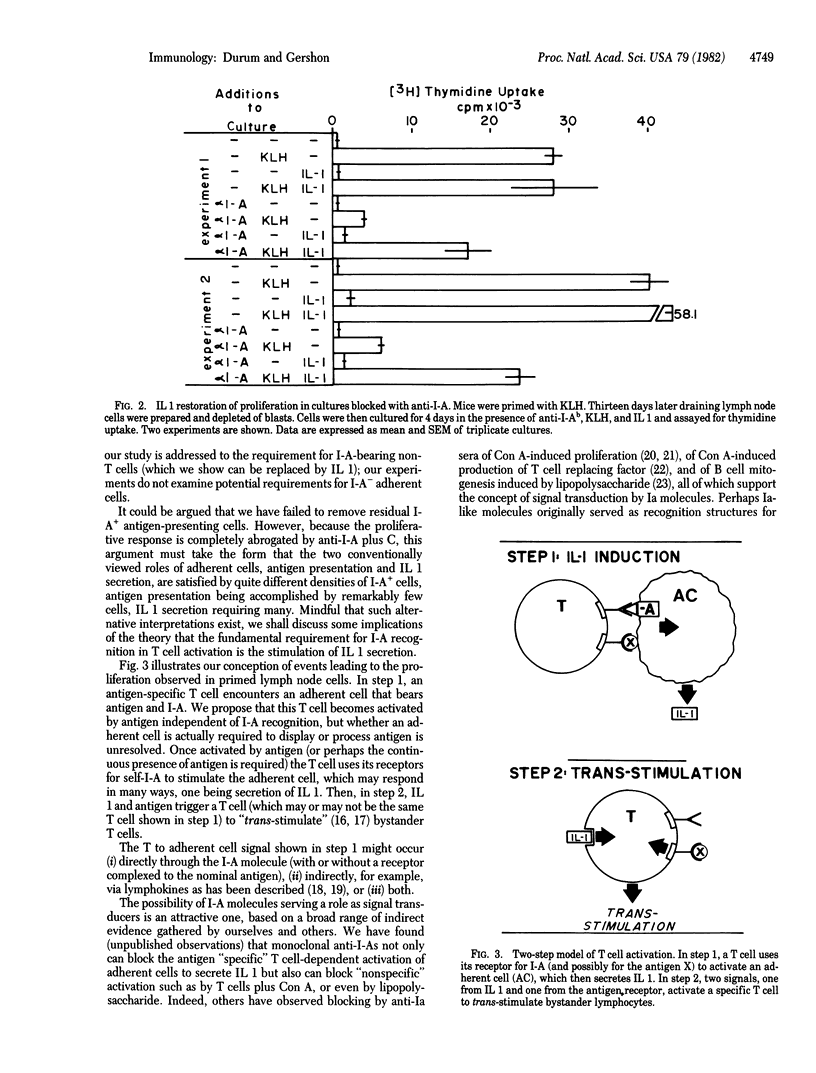
Antigen-primed T cells have been shown to require I-region-compatible adherent cells, as well as the priming antigen, to proliferate in vitro. We postulated that the Ia-recognition event is required for the T cell to induce secretion of the monokine interleukin 1 (IL 1) from adherent cells; the conventionally held view is that Ia is directly required for T cell activation. Our hypothesis predicts that IL I could replace the requirement for Ia+ cells in T cell proliferation assays in vitro. To test this prediction, we depleted keyhole limpet hemocyanin (KLH)-primed C57BL/6 mouse lymph node cells of I-A+ cells by treating with monoclonal anti-I-Ab and complement. As expected, this treatment eliminated the ability of KLH to provoke a proliferative response by primed T cells. Proliferation was restored by providing exogenous IL 1, but only in conjunction with added KLH. The proliferative response of primed T cells could also be blocked by adding anti-I-Ab to culture, and this inhibition could similarly be reversed by providing IL 1 in the presence of the specific antigen KLH. On the basis of these findings we propose a model of T cell activation and discuss its implications.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmann G. B., Sachs D. H., Hodes R. J. Requirement for an Ia-bearing accessory cell in Con A-induced T cell proliferation. J Immunol. 1978 Nov;121(5):1981–1989. [PubMed] [Google Scholar]
- Atkins E., Feldman J. D., Francis L., Hursh E. Studies on the mechanism of fever accompanying delayed hypersensitivity. The role of the sensitized lymphocyte. J Exp Med. 1972 May 1;135(5):1113–1132. doi: 10.1084/jem.135.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin A. A., Julius M. H., Cosenza H. Antigen-specific stimulation and trans-stimulation of T cells in long-term culture. Eur J Immunol. 1979 Sep;9(9):665–670. doi: 10.1002/eji.1830090903. [DOI] [PubMed] [Google Scholar]
- Burger R., Shevach E. M. Monoclonal antibodies to guinea pig Ia antigens. II. Effect on alloantigen-, antigen-, and mitogen-induced T lymphocyte proliferation in vitro. J Exp Med. 1980 Oct 1;152(4):1011–1023. doi: 10.1084/jem.152.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradin G., Etlinger H. M., Chiller J. M. Lymphocyte specificity to protein antigens. I. Characterization of the antigen-induced in vitro T cell-dependent proliferative response with lymph node cells from primed mice. J Immunol. 1977 Sep;119(3):1048–1053. [PubMed] [Google Scholar]
- Farr A. G., Dorf M. E., Unanue E. R. Secretion of mediators following T lymphocyte-macrophage interaction is regulated by the major histocompatibility complex. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3542–3546. doi: 10.1073/pnas.74.8.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gery I., Gershon R. K., Waksman B. H. Potentiation of cultured mouse thymocyte responses by factors released by peripheral leucocytes. J Immunol. 1971 Dec;107(6):1778–1780. [PubMed] [Google Scholar]
- Janeway C. A., Jr, Jason J. M. How T lymphocytes recognize antigen. Crit Rev Immunol. 1980 May;1(2):133–164. [PubMed] [Google Scholar]
- Kiertdzenbaum F., Waksman B. H. Mechanisms of action of 'lymphocyte activating factor' (LAF) I. Association of lymphocyte activating factor action with early DNA synthesis in PHA-stimulated lymphocytes. Immunology. 1977 Nov;33(5):663–669. [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Ersson B. Activation of T lymphocytes by lectins and carbohydrate-oxidizing reagents viewed as an immunological recognition of cell-surface modifications seen in the context of "self" major histocompatibility complex antigens. Eur J Immunol. 1981 Jun;11(6):475–483. doi: 10.1002/eji.1830110607. [DOI] [PubMed] [Google Scholar]
- Lachman L. B., Hacker M. P., Blyden G. T., Handschumacher R. E. Preparation of lymphocyte-activating factor from continuous murine macrophage cell lines. Cell Immunol. 1977 Dec;34(2):416–419. doi: 10.1016/0008-8749(77)90263-5. [DOI] [PubMed] [Google Scholar]
- Larsson E. L., Iscove N. N., Coutinho A. Two distinct factors are required for induction of T-cell growth. Nature. 1980 Feb 14;283(5748):664–666. doi: 10.1038/283664a0. [DOI] [PubMed] [Google Scholar]
- Matzinger P. A one-receptor view of T-cell behaviour. Nature. 1981 Aug 6;292(5823):497–501. doi: 10.1038/292497a0. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Ben-Zvi A. Studies on the role of lymphocyte-activating factor (Interleukin 1) in antigen-induced lymph node lymphocyte proliferation. Cell Immunol. 1980 Sep 1;54(2):382–389. doi: 10.1016/0008-8749(80)90218-x. [DOI] [PubMed] [Google Scholar]
- Mizel S. B. Studies on the purification and structure-functional relationships of murine lymphocyte activating factor (Interleukin 1). Mol Immunol. 1980 May;17(5):571–577. doi: 10.1016/0161-5890(80)90155-8. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Coppleson L. W. A THREE-CELL INTERACTION REQUIRED FOR THE INDUCTION OF THE PRIMARY IMMUNE RESPONSE in vitro. Proc Natl Acad Sci U S A. 1968 Oct;61(2):542–547. doi: 10.1073/pnas.61.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederhuber J. E., Frelinger J. A., Dugan E., Coutinho A., Shreffler D. C. Effects of anti-Ia serum on mitogenic responses. I. Inhibition of the proliferative response to B cell mitogen, LPS, by specific anti-Ia sera. J Immunol. 1975 Dec;115(6):1672–1676. [PubMed] [Google Scholar]
- Resch K., Heckmann B., Schober I., Bärlin E., Gemsa D. The role of macrophages in the activation of T lymphocytes by concanavalin A. III. Macrophage-independent activation. Eur J Immunol. 1981 Feb;11(2):120–126. doi: 10.1002/eji.1830110211. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. S., Shevach E. M. Function of macrophages in antigen recognition by guinea pig T lymphocytes. I. Requirement for histocompatible macrophages and lymphocytes. J Exp Med. 1973 Nov 1;138(5):1194–1212. doi: 10.1084/jem.138.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J., Caplan B., Paetkau V., Pilarski L. M., Delovitch T. L., McKenzie I. F. Cellular origins of co-stimulator (IL2) and its activity in cytotoxic T lymphocyte responses. J Immunol. 1980 May;124(5):2231–2239. [PubMed] [Google Scholar]
- Shevach E. M. The function of macrophages in antigen recognition by guinea pig T lymphocytes. III. Genetic analysis of the antigens mediating macrophage-T lymphocyte interaction. J Immunol. 1976 May;116(5):1482–1489. [PubMed] [Google Scholar]
- Singer A., Hathcock K. S., Hodes R. J. Self recognition in allogeneic radiation bone marrow chimeras. A radiation-resistant host element dictates the self specificity and immune response gene phenotype of T-helper cells. J Exp Med. 1981 May 1;153(5):1286–1301. doi: 10.1084/jem.153.5.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L. Role of Ia-positive cells in the production of T cell-replacing factors: blocking of factor production with anti-Ia serum. J Immunol. 1980 Sep;125(3):1224–1229. [PubMed] [Google Scholar]
- Tse H. Y., Schwartz R. H., Paul W. E. Cell-cell interactions in the T cell proliferative response. I. Analysis of the cell types involved and evidence for nonspecific T cell recruitment. J Immunol. 1980 Aug;125(2):491–500. [PubMed] [Google Scholar]
- Wagner H., Hardt C., Heeg K., Pfizenmaier K., Solbach W., Bartlett R., Stockinger H., Röllinghoff M. T-T cell interactions during cytotoxic T lymphocyte (CTL) responses: T cell derived helper factor (Interleukin 2) as a probe to analyze CTL responsiveness and thymic maturation of CTL progenitors. Immunol Rev. 1980;51:215–255. doi: 10.1111/j.1600-065x.1980.tb00323.x. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Callahan G. N., Althage A., Cooper S., Klein P. A., Klein J. On the thymus in the differentiation of "H-2 self-recognition" by T cells: evidence for dual recognition? J Exp Med. 1978 Mar 1;147(3):882–896. doi: 10.1084/jem.147.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]



