Abstract
Objective
To determine the effectiveness of a provider-based intervention to improve medication intensification among patients with diabetes.
Design
Effectiveness cluster-randomised trial. Baseline and follow-up cross-sections of diabetes physicians’ patients.
Setting
Eleven U.S. Southeastern states, 2006–2008.
Participants
205 Rural primary care physicians, 95 completed the study.
Intervention
Multicomponent interactive intervention including web-based continuing medical education (CME), performance feedback and quality improvement tools.
Primary outcome measures
Medication intensification, a dose increase of an existing medication or the addition of a new class of medication for glucose, blood pressure and lipids control on any of the three most recent office visits.
Results
Of 364 physicians attempting to register, 102 were randomised to the intervention and 103 to the control arms; 95 physicians (intervention, n=48; control, n=47) provided data on their 1182 of their patients at baseline (intervention, n=715; control, n=467) and 945 patients at follow-up (intervention, n=479; control, n=466). For A1c control, medication intensification increased in both groups (intervention, pre 26.4% vs post 32.6%, p=0.022; control, pre 24.8% vs post 31.1%, p=0.033) (intervention, adjusted OR (AOR) 1.37; 95% CI 1.06 to 1.76; control, AOR 1.41 (95% CI 1.06 to 1.89)); however, we observed no incremental benefit solely due to the intervention (group-by-time interaction, p=0.948). Among patients with the worst glucose control (A1c >9%), intensification increased in both groups (intervention, pre 34.8% vs post 62.5%, p=0.002; control, pre 35.7% vs post 61.4%, p=0.008).
Conclusions
A wide-reach, low-intensity, web-based interactive multicomponent intervention had no significant incremental effect on medication intensification for control of glucose, blood pressure or lipids for patients with diabetes of physicians practising in the rural Southeastern USA.
Trial registration
Keywords: Diabetes & Endocrinology, General Medicine (see Internal Medicine), Primary Care
Article summary.
Article focus
To determine the effectiveness of a wide-reach and low-intensity intervention aimed at providers to improve medication intensification among patients with diabetes in rural areas.
Key messages
In an effectiveness cluster-randomised trial among 205 rural physicians in 11 US states (Southeastern states; 2006–2008; 24 months), a wide-reach, low-intensity, web-based interactive multicomponent intervention had no significant incremental effect on medication intensification for control of glucose, blood pressure or lipids for patients with diabetes.
Medication intensification to control glucose increased in both the intervention and control groups and it was most significant at very poor levels of glucose control.
Strengths and limitations of this study
Strenghts include a designed cluster-randomised trial in a diverse rural area.
Limitations include the self-selection of charts for data abstraction. A high attrition rate may have introduced bias.
Introduction
Diabetes mellitus is highly prevalent in the USA.1 Diabetes research has produced clear evidence that blood glucose, lipid and blood pressure (BP) control forestall the vascular complications witnessed with uncontrolled diabetes. Thus, control of such diabetes care measures are well-accepted indicators—as intermediate process outcomes of high quality of care,2 3 However, diabetes care measures of their appropriate control lag behind; only 7% of diabetes patients in USA meet the American Diabetes Association recommendations for glycated haemoglobin (A1c), BP and low-density lipoprotein (LDL) control.4 Furthermore, diabetes is more endemic in the Southern USA and individuals with diabetes in this area have worse control.5–8 Hence, public health interventions, which consider distance barriers in rural setting and aimed at improving diabetes control in patients living in rural areas, are warranted.9–11
The Rural Diabetes Online Care (R-DOC) study was a rigorously tested cluster-randomised clinical trial comparing a multicomponent physician intervention including web-based continuous medical education (CME), performance feedback12–14 and quality improvement tools13 to a concurrent control website in rural Southeast USA.15 The primary outcomes were measures of acceptable and optimal control for A1c, BP and LDL; the secondary outcomes were process measures (rate of assessment and group means of A1c, BP and LDL). Despite the non-significant effect attributable to the intervention for any of the primary outcomes in the R-DOC study, we observed an absolute increase in the rates of assessment of A1c and LDL in both study arms (A1c, intervention 30%, control 29%, p<0.001; LDL, intervention 35%, control 38%, p=0.05).15 However, whether physicians in rural areas adjust medications to control A1c, BP or LDL has not been examined in wide-reach, low-intensity interventions9–11 such as the one utilised in the R-DOC study.
Thus, we now report the medication intensification outcomes of the R-DOC study—specifically, medication intensification to improve overall control of haemoglobin A1c, BP and LDL in the rural Southeastern USA. Our findings from the main R-DOC study and this report have important implications for distant CME programmes, quality improvement initiatives and implementation research.
Methods
Study design and setting
The R-DOC study was a cluster-randomised trial (ClinicalTrials.gov identifier: NCT00403091). The main study results—effect of the intervention on diabetes control—have been published elsewhere.15 Further details of the study design, web-content, recruitment and retention processes and patient characteristics have been published elsewhere.15–18 Briefly, participants were family, general and internal medicine physicians located in rural areas of 11 Southeastern US states (population size <25 000 habitants). Physicians were enrolled in the study sequentially from September 2006 to September 2008, provided informed consent online, and were randomised to the intervention or control arms using block randomisation which was concealed to the investigators and statisticians. The unit of randomisation was the physician (and not a practice); however, for physicians working in a group practice, only one physician per practice could enrol in the study. The protocol was approved by the local institutional review boards at The University of Alabama at Birmingham and Tuscaloosa medical campuses.
Intervention arm description
The website focused on helping physicians to achieve A1c, BP and LDL control in their diabetic patients.15 18 Specifically, the intervention site contained: (1) challenging cases; (2) individualised diabetes performance feedback reports based on the physician's own panel of patients; (3) practice timesavers; (4) practical goals and guidelines, including guidance for quality improvement and systems redesign;13 (5) patient resources; and (6) an area to track and view CME credit. Intervention arm physicians also received tailored email reminders about website updated and uncompleted sections of the website.15
Individualised performance feedback reports were based on physician's patients with diabetes. The feedback reports compared each intervention arm participant's personal performance compared to the performance for the top 10% of other intervention arm participants.12 The feedback reports consisted of diabetes control (A1c <7%, systolic BP <130 mm Hg, LDL <100 mg/dl), counselling on diet or exercise and medication intensification.15
Control arm description
The control website contained: (1) links to diabetes practice guidelines and patient education materials; (2) a list of educational conferences on general medical topics (updated monthly); (3) an area to track and view their CME credit and (4) a link to an external medical blog. Physicians in the control group did not receive performance feedback reports or electronic communications.
Data sources
All participating physicians provided copies of records of 15 (intervention arm physicians) or 10 (control arm physicians) of their own consecutively seen patients with diabetes at baseline and again at follow-up (representing two cross-sectional views of each physician's panel of patients). Since the focus of the intervention was the physician, we were less interested in specific patients but rather each physician's panel on average. Therefore, two samples as serial cross-sections would better represent any change in the physician's own panel on average. This method is similar to practice feedback for quality improvement purposes.19 20 The number of records was selected to balance rigour and cost (see also sample size description in the statistical section below).
Patient inclusion criteria were having at least two office visits during the past year and no dialysis, dementia, organ transplantation, HIV/AIDS, terminal illness or malignancy (except for skin and prostate cancer). Data abstraction was performed by trained personnel on blinded records sent to the study centre (or abstracted on site). Details of data abstracted, quality controls and physician compensation are published elsewhere.15
Outcomes
In our main study,15 the main outcomes were measures of acceptable and optimal diabetes control.2 3 The main outcome for this report is medication intensification, defined as a dose increase of an existing medication or the addition of a new class of medication for glucose, blood pressure and lipids control on any of the three most recent office visits. Medication intensification equalled the proportion of patients who had a glucose-lowering medication, antihypertensive medication or cholesterol-lowering medication added or increased over the three most recent visits divided by the total number of patients assessed at baseline or follow-up. As a secondary outcome, we also explored medication intensification by level of glucose, BP or lipid control.
Statistical approach
We calculated the sample size for the main study results.15 On the basis of clinically important differences, the study required 100 physicians per trial arm to detect a minimum of 0.4% difference in A1c, 6 mm Hg in BP and 6 mg/dl in LDL for the main outcomes (power 80%, α=0.05, up to 20% dropout) (10 patients per physician at baseline and at follow-up).15 However, intervention arm physicians were asked to provide 15 patient records at baseline (and not at follow-up) because of the need to construct audit and feedback reports as part of the intervention19 20 for A1c, BP and lipids, reflecting the fact that not all diabetes patients also have hypertension and hyperlipidaemia. The initial study was not powered to perform adjusted analysis for the intensification outcomes of this report.
All analyses followed the intention-to-treat principle. The relationship between the main and secondary outcomes and the effect of the intervention were examined with generalied linear mixed models (GLMM), accounting for clustering of patients within physicians. ORs for follow-up versus baseline within the two groups were calculated, adjusting for covariates that differed between baseline and follow-up patient populations; thus, the covariates included in these adjusted analyses included race, and clinical diagnosis of hypertension or depression. GLMM was implemented by PROC GLIMMIX in SAS and was adjusted for race, and clinical diagnosis of hypertension or depression. Thus, the group coefficient represented differences between the intervention and control groups at baseline; the time of coefficient represented changes in the control group over time, thus capturing temporal trends. Finally, the group-by-time interaction coefficient represented the difference-of-differences for over-time change in the intervention versus control group, and was a direct comparison of over-time change for the intervention versus the control group. A positive OR meant that the odds of a patient being in control increased more over time for the intervention versus control group. All analyses were performed in SAS V.9.2 (Cary, North Carolina, USA).
Results
Recruitment scheme, patient characteristics and web utilisation
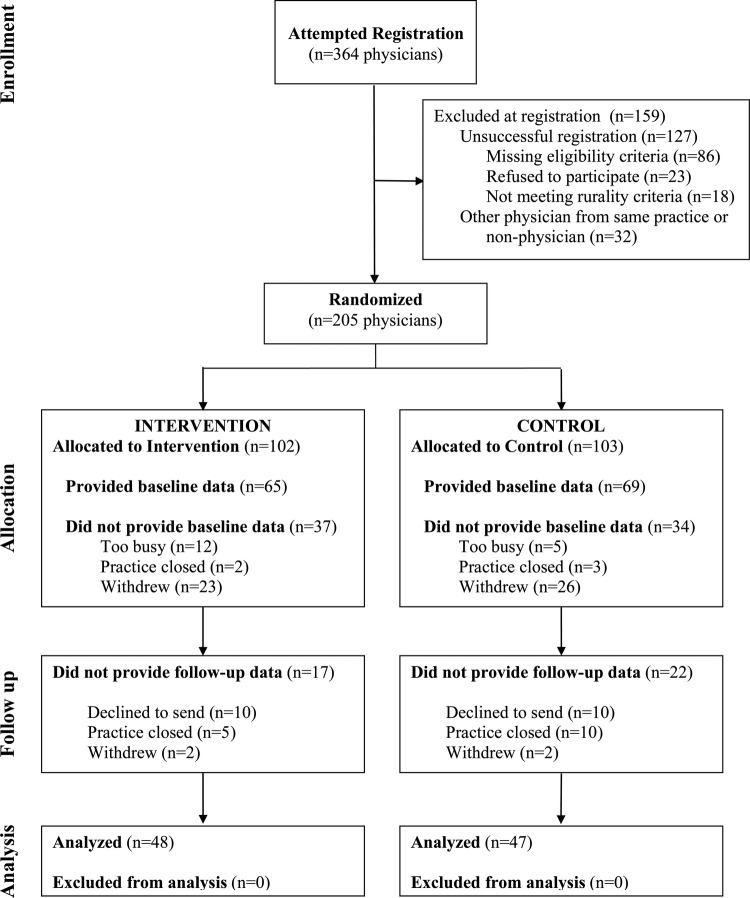
The Consolidated Standards of Reporting Trials diagram and patients characteristics at baseline and at follow-up (modified from a prior publication15) are included in this report for completeness (figure 1 and table 1). We obtained baseline and follow-up data on 95 physicians (intervention, n=48; control, n=47) and 1182 of their patients at baseline (intervention, n=715; control, n=467) and 945 diabetes patients at follow-up (intervention, n=479; control, n=466).
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram (a prior version of this figure has been reported, reproduced with permission15).
Table 1.
Characteristics of diabetes patients of 95 physicians randomised to intervention (n=48) or control (n=47) who completed follow-up
| Patient characteristic | Baseline |
Follow-up |
||||
|---|---|---|---|---|---|---|
| Intervention (n=715) | Control (n=467) | Total (n=1182) | Intervention (n=479) | Control (n=466) | Total (n=945) | |
| Age (years) | 58.7 (13.59) | 60.6 (13.79) | 59.4 (13.70) | 61.3 (13.42) | 60.5 (12.71) | 60.9 (13.09) |
| Gender, female | 360 (51.1%) | 230 (49.5%) | 590 (50.5%) | 260 (54.5%) | 252 (54.4%) | 512 (54.5%) |
| Race, African American | 97 (13.9%) | 99 (21.3%) | 196 (16.9%) | 102 (21.4%) | 143 (30.9%) | 245 (26.1%) |
| Obesity* | 274 (38.3%) | 193 (41.3%) | 467 (39.5%) | 97 (20.3%) | 75 (16.1%) | 172 (18.2%) |
| Smoker, current | 85 (12.5%) | 55 (12.3%) | 140 (12.4%) | 63 (13.8%) | 53 (13.0%) | 116 (13.4%) |
| No self-testing | 168 (33.6%) | 114 (36.3%) | 282 (34.6%) | 189 (41.4%) | 183 (44.7%) | 372 (43.0%) |
| Non-adherence to appointments | 75 (11.0%) | 54 (12.0%) | 129 (11.4%) | 10 (2.2%) | 23 (5.6%) | 33 (3.8%) |
| Insurance, Medicaid | 65 (9.1%) | 51 (10.9%) | 116 (9.8%) | 66 (13.8%) | 58 (12.4%) | 124 (13.1%) |
| Comorbidities | ||||||
| Diabetes complications† | 147 (20.6%) | 110 (23.6%) | 257 (21.7%) | 156 (32.6%) | 134 (28.8%) | 290 (30.7%) |
| Insulin use | 103 (14.4%) | 66 (14.1%) | 169 (14.3%) | 84 (17.5%) | 68 (14.6%) | 152 (16.1%) |
| Hypertension | 473 (66.2%) | 304 (65.1%) | 777 (65.7%) | 327 (68.3%) | 283 (60.7%) | 610 (64.6%) |
| Hyperlipidaemia | 110 (15.4%) | 85 (18.2%) | 195 (16.5%) | 11 (2.30%) | 17 (3.7%) | 28 (3.0%) |
| Peripheral vascular disease | 47 (6.6%) | 31 (6.6%) | 78 (6.6%) | 41 (8.6%) | 35 (7.5%) | 76 (8.0%) |
| Coronary artery disease | 135 (18.9%) | 83 (17.8%) | 218 (18.4%) | 111 (23.2%) | 95 (20.4%) | 206 (21.8%) |
| Vascular intervention‡ | 60 (8.4%) | 47 (10.1%) | 107 (9.1%) | 36 (7.5%) | 40 (8.6%) | 76 (8.0%) |
| Depression | 105 (14.7%) | 61 (13.1%) | 166 (14.0%) | 84 (17.5%) | 60 (12.9%) | 144 (15.2%) |
A prior version of this table has been reported (reproduced with permission).15
Values are mean (SD) or n (%).
*Obesity: body mass index ≥ 30 kg/m2 or clinical diagnosis.
†Diabetes complications: retinopathy, neuropathy or nephropathy.
‡Vascular intervention: coronary artery bypass grafting, stent, percutaneous coronary angioplasty.
At baseline, intervention group physicians provided records for fewer African American patients and more patients with hypertension and depression compared with control group physicians. Of the 95 physicians, 90 (94.7%) had access to the Internet in the office.
Main outcomes: medication intensification
In the adjusted analysis, we saw no significant effect attributable to the intervention in the group-by-time interaction term for any of the medication intensification measures (A1c, BP and LDL; p=0.948, 0.216 and 0.995; respectively).
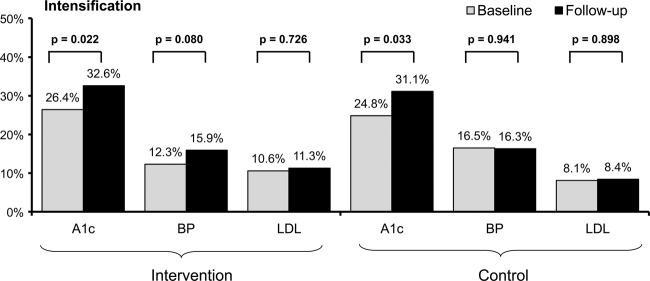
In the unadjusted analysis, intensification of medications to control A1c increased in both trial arms when baseline and follow-up data were compared (intervention, 26.4% vs 32.6%, p=0.022; control, 24.8% vs 31.1%, p=0.033; figure 2). This was confirmed in the adjusted analysis (intervention, adjusted OR (AOR) 1.37; 95% CI 1.06 to 1.76; control, AOR 1.41 (95% CI 1.06 to 1.89)).
Figure 2.
Medication intensification for haemoglobin A1c (%), blood pressure (BP, mm Hg) and cholesterol (low-density lipoprotein (LDL), mg/dl) control for intervention (n=48) or control (n=47) physicians, at baseline (pre, n=1182 patients) and follow-up (post, n=945 patients). See text for definitions of medication intensification.
In the unadjusted analysis, intensification of medications to control BP did not differ for patients cared for by physicians in either trial arm when comparing baseline and follow-up data (intervention, 12.3% vs 15.9%, p=0.080; control, 16.5% vs16.3%, p=0.941; figure 2). This finding was consistent with the adjusted analysis (intervention, AOR 1.37 (95% CI 0.99 to 1.88); control, AOR 1.00 (95% CI 0.72 to 1.41)).
In the unadjusted analysis, intensification of medications to control LDL did not differ for patients cared for by physicians in either trial arm when comparing baseline and follow-up data (intervention, 10.6% vs 11.3%, p=0.726; control, 8.1% vs 8.4%, p=0.898; figure 2). This finding was consistent with the adjusted analysis (intervention, AOR 1.05 (95% CI 0.73 to 1.50); control, AOR 1.00, (95% CI 0.65 to 1.53)).
Secondary outcome: medication intensification by strata
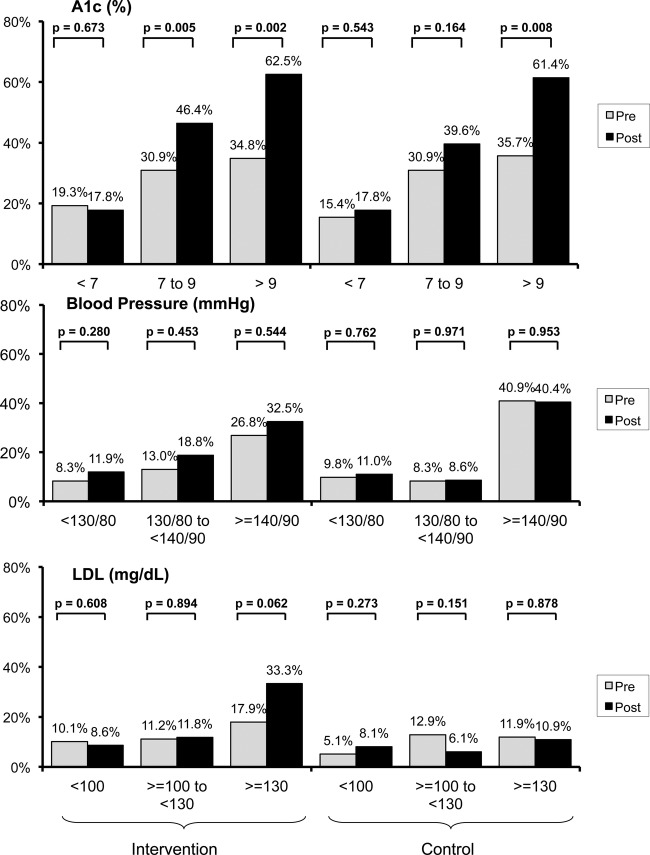
In the unadjusted analysis and among patients with worse glucose control (A1c >9%), intensification of medications to control A1c increased in both trial arms between baseline and follow-up (intervention, pre 34.8% vs post 62.5%, p=0.002; control, pre 35.7% vs post 61.4%, p=0.008; figure 3 top panel). For patients whose last A1c ≥7 to ≤9, we noted an increase in intensification between the baseline and follow-up for the intervention group (p=0.005) but no difference in the control group (p=0.164; figure 3 top panel). Among patients with best glucose control (A1c <7%), medication intensification between baseline and follow-up was similar for both trial arms (intervention, p=0.673; control, p=0.543; figure 3 top panel).
Figure 3.
Medication intensification for strata of haemogloblin A1c (%), blood pressure (BP, mm Hg) and cholesterol (low-density lipoprotein (LDL), mg/dl) control. See text for definitions of medication intensification.
In the unadjusted analysis, intensification of BP medication between baseline and follow-up was similar at all levels of BP control for both trial arms (figure 3 middle panel).
Similarly, in the unadjusted analysis, intensification of lipid-lowering medications between baseline and follow-up was similar at all levels of LDL control for both trial arms (figure 3 bottom panel).
Discussion
In a rigorously designed cluster-randomised trial, a wide-reach, low-intensity web-based multicomponent intervention for primary care physicians in the rural Southern USA had no significant incremental effect on medication intensification for their diabetes patients’ A1c, BP or LDL control as compared to a concurrent control website focusing on diabetes care. However, the absolute rate of intensification of medications to control A1c increased in both study arms at the end of the study by a modest amount (∼6%). Although the study was not designed to perform subgroup analysis, the rate of intensification was greatest among the worst-controlled patients (A1c >9%) in both study arms (∼25–28%). Plausible explanations for our findings include the low engagement with the intervention, higher-than anticipated attrition rate, Hawthorne's effect or no effect of the intervention15 18
Medication intensification is emerging as a process measure for quality improvement efforts among patients with diabetes mellitus; pharmacotherapy intensification is strongly linked to improved A1c, BP and LDL. For example, in a study of over one million members with hypertension, hyperlipidaemia and diabetes mellitus at Kaiser Permanente Northern California, Selby et al21 found that a 5% improvement in treatment intensification led to a 1.0–1.9% improvement in control for the entire population. However, the association of treatment intensification rates to improved risk factor control in rural populations has not been examined. Further evidence and reviews of the importance of medication intensification among patients with diabetes are available elsewhere22–27.
Trials using medication intensification outcomes
Relatively few studies involving physicians have examined medication intensification as an outcome, none in a rural setting. Studies have tested the impact of a simulated case-based education with opinion leader feedback,28 customised feedback29 and real-time feedback to patients and their physicians using cell phone software;30 these studies are summarised below.
In a group randomised controlled trial28 of 57 primary care physicians and 2020 patients in an urban setting, physicians were randomised to no intervention, a simulated case-based physician learning intervention, or the same case-based and leader feedback. Glucose control, mean A1c value, worsened in the control group by 0.06, improved in the simulated case-based group by 0.01, and worsened in the case-based and leader feedback by 0.18. Among the 907 patients with A1c>7%, medication intensification was similar between groups (31.5%, control; 32.6%, case-based; 36.8%, case-based and leader feedback; p=0.41). Similarly, among the 701 patients with LDL-C >99 mg/dl, medication intensification was similar between groups (20.8%, control; 24.2%, case-based; 21.8%, case-based and leader feedback; p=0.66). Finally, among the 949 patients with BP values >130/mm Hg, medication intensification was similar between groups (27.3%, control; 24.7%, case-based; 28.2%, case-based and leader feedback; p=0.61). Medication intensification was defined by the initiation or titration in 12 months after the intervention for all patients with goals above target (A1c>=7%, LDL-C >=100 mg/dl, BP>130/80 mm Hg); insulin titration was excluded from the outcome. The attrition rate was 33% (38/59 physicians). Our case scenarios took less than 10 min to complete as compared with the ones in this study (over 60 min each).28 Perhaps striking a balance between more ‘intense’ cases to increase physician engagement and providing feedback information may lead to the improvement in medication intensification. Finally, the medication intensification rates definitions were different from ones used in our study; hence, they cannot be compared.
In a randomised trial of 3703 patients with diabetes and their 123 physicians, participants were randomised to receive customised feedback of clinical information in four groups: patient only, physician only, both the patient and physician, or neither one.29 At 12 months, interventions had no effect on A1c test ordering, medication intensification or improvement in A1c or LDL control. Medication intensification was defined as in the above study.28 In our study, we included customised feedback as one of the many components of the intervention.
In a pilot-controlled trial,30 30 patients with diabetes were randomised to an intervention group consisting of cell phone-based software which provided real-time feedback on glucose levels, medication regimens and suggested treatment algorithms or a control group. Intervention providers received patients’ logbooks and suggested treatment plans to reach A1c<6.5%. During the 3 months of the study, the A1c levels decreased among patients in the intervention as compared with the control group (by 2.0% vs 0.7%; respectively); medications were changed or intensified in 84% in the intervention group as compared with 23% patients in the control group (p<0.002). The operational definition for medication intensification in this study was not reported. Cell phone technology provides the opportunity for wide reach approach. In our study, physicians and not their patients received the customised feedback on their group of patients.
Limitations
Our study had limitations.15 First, physicians themselves provided the records of consecutively seen patients; although selection bias was a possibility, the wide range of A1c, BP and LDL values suggests that not only well-controlled patients were selected. Second, the high attrition, 95 of the 205 randomised physicians provided baseline and follow-up data, may have introduced biases. By design, to test a purely online intervention, we chose not to enhance retention activities as typically seen in randomised clinical trials and focused on several aspects of provider-based implementation tools.13 Although a comprehensive analysis would have been enlightening, we did not perform a systematic examination of this negative trial, or how such intervention might work it were effective. We worried that studying the study while being conducted would have introduced another variable.
Implications
A framework has been proposed to evaluate the public health impact of interventions.9–11 The components of the framework include reach (‘How many participate?’), effectiveness (‘Does it work in usual settings?’), adoption (‘How many use it?’), implementation (‘Is it used as intended?’) and maintenance (‘Is it sustained over time?’) (RE-AIM). In this study, using a wide-reach approach, we examined the effectiveness of a potential public health intervention. Effective interventions may be limited by their low-reach, specificity to fewer settings, expense and limited sustainability—thus limiting their generalisability. Testing less intensive interventions with wider reach in rural settings has been advocated in public health interventions.11 Finding effective strategies to accomplish continuing professional development for physicians practising in remote locations is also an important objective.31 In contrast, higher-intensity interventions in rural and community settings have shown promising results.32–36
Conclusion
In conclusion, a wide-reach, low-intensity, web-based interactive multicomponent intervention had no significant incremental effect on medication intensification for control of glucose, BP or lipids for patients with diabetes of physicians practising in the rural Southeastern USA. Despite low engagement in the web-based programme, intensification measures improved in both arms of the study. Our study raises important issues for wide-reach, low-intensity CME programmes that seek to improve patient outcomes.
Supplementary Material
Footnotes
Contributors: KB wrote the article and contributed in the interpretation of the findings. YK conducted the analyses and contributed to the writing of the Methods and Results sections. MMS, AHS, TKH, WC, JJA, CAE contributed to the conceptualisation of the project, the interpretation of the findings and to the writing of all sections of the article. JJA conceived and supervised the study. All authors contributed to reviewing drafts of the article.
Funding: This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) 5R18DK065001 to JJA. AHS and CAE were supported by the Veterans Affairs National Quality Scholars Program. The sponsors had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript.
Competing interests: The opinions expressed in this article are those of the authors alone and do not reflect the views of the Department of Veterans Affairs.
Ethics approval: Institutional review boards at The University of Alabama at Birmingham and Tuscaloosa medical campuses.
Provenance and peer review: Not commissioned; externally peer reviewed
Data sharing statement: No additional data are available.
References
- 1.Centers for Disease Control and Prevention National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2008 [Google Scholar]
- 2.American Diabetes Association Standards of medical care in diabetes–2006. Diabetes Care 2006;29(Suppl 1):S4–42 [PubMed] [Google Scholar]
- 3.National Committee for Quality Assurance Comprehensive diabetes care. HEDIS 2009 volume 2 technical update, 2008
- 4.Weitzman S, Greenfield S, Billimek J, et al. Improving combined diabetes outcomes by adding a simple patient intervention to physician feedback: a cluster randomized trial. Isr Med Assoc J 2009;11:719–24 [PubMed] [Google Scholar]
- 5.Jencks SF, Cuerdon T, Burwen DR, et al. Quality of medical care delivered to Medicare beneficiaries: a profile at state and national levels. JAMA 2000;284:1670–6 [DOI] [PubMed] [Google Scholar]
- 6.Weingarten JP, Jr, Brittman S, Hu W, et al. The state of diabetes care provided to Medicare beneficiaries living in rural America. J Rural Health 2006;22:351–8 [DOI] [PubMed] [Google Scholar]
- 7.Andrus MR, Kelley KW, Murphey LM, et al. A comparison of diabetes care in rural and urban medical clinics in Alabama. J Community Health 2004;29:29–44 [DOI] [PubMed] [Google Scholar]
- 8.Krishna S, Gillespie KN, McBride TM. Diabetes burden and access to preventive care in the rural United States. J Rural Health 2010;26:3–11 [DOI] [PubMed] [Google Scholar]
- 9.Glasgow RE, Emmons KM. How can we increase translation of research into practice? Types of evidence needed. Annu Rev Public Health 2007;28:413–33 [DOI] [PubMed] [Google Scholar]
- 10.Glasgow RE, Klesges LM, Dzewaltowski DA, et al. Evaluating the impact of health promotion programs: using the RE-AIM framework to form summary measures for decision making involving complex issues. Health Educ Res 2006;21:688–94 [DOI] [PubMed] [Google Scholar]
- 11.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health 1999;89:1322–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weissman NW, Allison JJ, Kiefe CI, et al. Achievable benchmarks of care: the ABCs of benchmarking. J Eval Clin Pract 1999;5:269–81 [DOI] [PubMed] [Google Scholar]
- 13.Salanitro AS, Estrada CA, Allison JJ. Implementation research: beyond the traditional randomized controlled trial. In: Glasser S, ed. Essentials of clinical research. New York, NY: Springer Science, 2008:217–44 [Google Scholar]
- 14.Kiefe CI, Allison JJ, Williams OD, et al. Improving quality improvement using achievable benchmarks for physician feedback: a randomized controlled trial. JAMA 2001;285:2871–9 [DOI] [PubMed] [Google Scholar]
- 15.Estrada CA, Safford MM, Salanitro AH, et al. A web-based diabetes intervention for physician: a cluster-randomized effectiveness trial. Int J Qual Health Care 2011;23:682–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster PP, Williams JH, Estrada CA, et al. Recruitment of rural physicians in a diabetes internet intervention study: overcoming challenges and barriers. J Natl Med Assoc 2010;102:101–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Safford MM, Shewchuk R, Qu H, et al. Reasons for not intensifying medications: differentiating ‘clinical inertia’ from appropriate care. J Gen Intern Med 2007;22:1648–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crenshaw K, Curry W, Salanitro AH, et al. Is physician engagement with Web-based CME associated with patients’ baseline hemoglobin A1c levels? The Rural Diabetes Online Care study. Acad Med 2010;85:1511–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allison JJ, Calhoun JW, Wall TC, et al. Optimal reporting of health care process measures: inferential statistics as help or hindrance? Manag Care Q 2000;8:1–10 [PubMed] [Google Scholar]
- 20.Holmboe ES, Meehan TP, Lynn L, et al. Promoting physicians’ self-assessment and quality improvement: the ABIM diabetes practice improvement module. J Contin Educ Health Prof 2006;26:109–19 [DOI] [PubMed] [Google Scholar]
- 21.Selby JV, Uratsu CS, Fireman B, et al. Treatment intensification and risk factor control: toward more clinically relevant quality measures. Med Care 2009;47:395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zikmund-Fisher BJ, Hofer TP, Klamerus ML, et al. First things first: difficulty with current medications is associated with patient willingness to add new ones. Patient 2009;2:221–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bebb C, Kendrick D, Coupland C, et al. A cluster randomised controlled trial of the effect of a treatment algorithm for hypertension in patients with type 2 diabetes. Br J Gen Pract 2007;57:136–43 [PMC free article] [PubMed] [Google Scholar]
- 24.Carter BL, Bergus GR, Dawson JD, et al. A cluster randomized trial to evaluate physician/pharmacist collaboration to improve blood pressure control. J Clin Hypertens (Greenwich) 2008;10:260–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant R, Adams AS, Trinacty CM, et al. Relationship between patient medication adherence and subsequent clinical inertia in type 2 diabetes glycemic management. Diabetes Care 2007;30:807–12 [DOI] [PubMed] [Google Scholar]
- 26.Rodondi N, Peng T, Karter AJ, et al. Therapy modifications in response to poorly controlled hypertension, dyslipidemia, and diabetes mellitus. Ann Intern Med 2006;144:475–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heisler M, Hogan MM, Hofer TP, et al. When more is not better: treatment intensification among hypertensive patients with poor medication adherence. Circulation 2008;117:2884–92 [DOI] [PubMed] [Google Scholar]
- 28.O'Connor PJ, Sperl-Hillen JM, Johnson PE, et al. Simulated physician learning intervention to improve safety and quality of diabetes care: a randomized trial. Diabetes Care 2009; 32:585–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Connor PJ, Sperl-Hillen J, Johnson PE, et al. Customized feedback to patients and providers failed to improve safety or quality of diabetes care: a randomized trial. Diabetes Care 2009;32:1158–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinn CC, Clough SS, Minor JM, et al. WellDoc mobile diabetes management randomized controlled trial: change in clinical and behavioral outcomes and patient and physician satisfaction. Diabetes Technol Ther 2008;10:160–8 [DOI] [PubMed] [Google Scholar]
- 31.Curran V, Rourke L, Snow P. A framework for enhancing continuing medical education for rural physicians: a summary of the literature. Med Teach 2010;32:e501–8 [DOI] [PubMed] [Google Scholar]
- 32.Maclean CD, Gagnon M, Callas P, et al. The Vermont diabetes information system: a cluster randomized trial of a population based decision support system. J Gen Intern Med 2009;24:1303–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan S, Maclean CD, Littenberg B. The effect of the Vermont Diabetes Information System on inpatient and emergency room use: results from a randomized trial. Health Outcomes Res Med 2010;1:e61–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beem SE, Machala M, Holman C, et al. Aiming at ‘de feet’ and diabetes: a rural model to increase annual foot examinations. Am J Public Health 2004;94:1664–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayer-Davis EJ, D'Antonio AM, Smith SM, et al. Pounds off with empowerment (POWER): a clinical trial of weight management strategies for black and white adults with diabetes who live in medically underserved rural communities. Am J Public Health 2004;94:1736–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spencer MS, Rosland AM, Kieffer EC, et al. Effectiveness of a community health worker intervention among African American and Latino adults with type 2 diabetes: a randomized controlled trial. Am J Public Health 2011;101:2253–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.