Abstract
Objectives
Dementia prevalence and its burden on families are increasing. Caregivers of persons with dementia have more depression and stress than the general population. Several interventions have proven efficacy in decreasing depression and stress in selected populations of caregivers. Hispanics in New York City tend to have a higher burden of dementia caregiving compared to non-Hispanic whites (NHW) because Hispanics have a higher prevalence of dementia, tend to have high family involvement, and tend to have higher psychosocial and economic stressors. Thus, we chose to test the effectiveness of a dementia caregiving intervention, the New York University Caregiver Intervention (NYUCI), with demonstrated efficacy in spouse caregivers in Hispanic relative caregivers of persons with dementia. Including the community health worker (CHW) intervention in both arms alleviates general psychosocial stressors and allows the assessment of the effectiveness of the intervention. Compared to two original efficacy studies of the NYUCI, which included only spouse caregivers, our study includes all relative caregivers, including common law spouses, children, siblings, a nephew and nieces. This study will be the first randomised trial to test the effectiveness of the NYUCI in Hispanic caregivers including non-spouses.
Methods and analysis
The design of the study is a randomised controlled trial (RCT). Participants are randomised to two arms: case management by a CHW and an intervention arm including the NYUCI in addition to case management by the CHW. The duration of intervention is 6 months. The main outcomes in the trial are changes in the Geriatric Depression Scale (GDS) and the Zarit Caregiver Burden Scale (ZCBS) from baseline to 6 months.
Ethics and dissemination
This trial is approved by the Columbia University Medical Center Institutional Review Board (AAAI0022), and funded by the National Institute on Minority Health and Health Disparities. The funding agency has no role in dissemination.
Trial Registration
www.ClinicalTrials.gov NCT01306695.
Keywords: Mental Health
Article summary.
Article focus
This article describes the rationale and protocol of an ongoing randomised trial testing a counselling intervention in Hispanic caregivers of persons with dementia.
Key messages
Our project is a randomised controlled trial (RCT).
Our project is an example of comparative effectiveness research (CER).
This study is the first randomised trial testing the NYU caregiver intervention in Hispanic caregivers.
Strengths and limitations of this study
Strengths include the RCT design and rigorous analysis plan.
Relative limitations are relatively short duration and lack of power for subgroup analyses.
Introduction
Burden of dementia
Dementia is an epidemic in our ageing society. Dementia is a syndrome characterised by impairment of memory and other cognitive abilities severe enough to cause functional impairment (ie, the ability to live independently).1 Dementia in this article refers to late-onset sporadic dementia, occurring mostly in persons 65 years and older. The most common dementia is Alzheimer's dementia (AD),2 3 comprising between 70% and 90% of cases. The second most-common cause is vascular dementia, caused by stroke,4 comprising between 5% and 25% of cases of dementia. Other causes of dementia such as fronto-temporal dementia and Lewy body disease are less common (<5% of cases).3 Dementia prevalence increases after the age of 70 years,5 and its prevalence may reach 50% in persons 85 years and older.6 In 2011, the Alzheimer's Association estimated that 5.4 million people have AD (one in eight older Americans), taken care of by 14.9 million unpaid caregivers, resulting in 183 billion dollars in annual costs.3 The prevalence of AD is expected to increase to 5.7 million in 2020, 7.7 million in 2030 and 11 million in 2040. Consequently, the number of family caregivers is increasing. Despite our increasing understanding of dementia, particularly AD, no current known preventive or curative measure exists.7 AD is the sixth leading cause of death in the USA, and the fifth for those aged 65 years and older.3 In this context, dementia prevalence and the number of family caregivers continues to increase with the ageing of the population.
Burden of dementia caregiving
Caring for a person with AD or another dementia is challenging. In addition to memory loss, dementia impairs judgement, orientation and the ability to comprehend and communicate. Personality and behaviour are affected as well.8–10 Individuals with dementia require increasing levels of supervision and personal care as the disease progresses, and many family caregivers experience isolation, high levels of stress and problems with health, employment, income and financial security. The responsibilities associated with caring for a family member with dementia places caregivers at risk for psychological and physical illness.11 Although caregivers may draw some satisfaction from caregiving, they report high levels of emotional stress and depression.12 13 Family caregivers of people with AD and other dementia report significant caregiving strain concerning financial issues.3 Caregiver stress related to the impaired person's behavioural symptoms often leads to nursing home placement (NHP).13 14 However, even after caregivers place their family member in a nursing home, many still report high levels of emotional and physical stress.15 16 While three-quarters of family caregivers of people with AD and other dementias report no guilt in deciding to place their family member in a nursing home,3 this is less common among Hispanics.3 17
Dementia caregiving in Hispanics
The prevalence of dementia in Hispanics is higher than in non-Hispanic whites (NHW) nationally (27.9% vs 10.9% in persons aged 75–84 years; 62.9% vs 30.2% in persons 85 years and older), including New York City.18 19 Moreover, Hispanics are the fastest-growing ethnic group in the USA in general,20 and in the age groups of >65 and >85 years, the groups most at risk for cognitive impairment and dementia, respectively. It is not surprising that the Alzheimer's Association has estimated that the ethnic group with the fastest-growing prevalence of dementia is Hispanics.21 This disparity does not seem to be explained by bias in diagnosis due to language or education but may be explained by a higher burden of risk factors for dementia in Hispanics compared to NHW, such as diabetes.18 Consequently, the burden of dementia care is proportionally higher in Hispanics compared with NHW. In addition, Hispanics tend to delegate less of the care of their relatives with dementia to paid caregivers.17
According to surveys conducted by the Alzheimer's Association, Hispanic caregivers are on average 43 years old, and younger than NHW, and African-American caregivers.3 They are less likely to be married than NHW caregivers (48% vs 63%) and more likely to have children or grandchildren under age 18 living in their household (47% vs 32% of all caregivers, 30% of NHW caregivers and 30% of African-American caregivers). Hispanic caregivers are more likely to be a primary caregiver (61% contrasted with 48% of NHW caregivers and 43% of Asian-American caregivers) and more likely to report an annual income of under US$50 000 (56% vs 39% of caregivers overall, 34% of NHW caregivers and 31% of Asian-American caregivers). They are more likely to report needing help balancing their work and family responsibilities (39% vs 27% of caregivers overall and 25% of NHW caregivers) and finding time for themselves (41% vs 29% of NHW caregivers).3 In summary, the caregiving experience tends to be accompanied by more stressors for Hispanics compared to NHW.
The New York University Caregiver Intervention
The NYU Caregiver Intervention (NYUCI) has substantial evidence of efficacy for spouse caregivers of persons with AD and related dementias.22–31 The underlying theme of the NYUCI is that improving social support improves caregiver well-being, and thereby obviates or defers the need for NHP.22 23 25 The NYUCI was evaluated in a longitudinal randomised controlled trial (RCT) over more than 2 decades that included 406 spouse caregivers. The intervention alleviated the deleterious effects of caregiving on mental and physical health of spouse caregivers,22–24 and postponed or prevented NHP of their AD patient spouses.25 26 The NHP rate of people with dementia whose spouses received the NYUCI was 72% of the NHP rate in the usual care group, and the median difference between the two groups was 557 days.26 Moreover, the intervention's effects on caregiver depression were long lasting and continued through NHP and death of the person with AD.27–30 Changes in caregiver reaction to the spouse's memory and behavioural problems, satisfaction with social support and depression collectively explained 61.2% of the intervention's effect on NHP of their spouses.26 A mediation analysis demonstrated that a substantial proportion of effect on change in these outcomes could be attributed to intervention-induced increases in the caregivers’ satisfaction with their social support networks.29 The intervention increased both objective indicators of social support, and more subjective measures. The effects of change in satisfaction with social support were found to be significant predictors of both change in stress appraisals (p<0.0001) and change in depression (p<0.0001).29 The NYUCI is listed and described in detail on the National Registry of Evidence-based Programs and Practices website.31 This intervention is unique in its emphasis on family support and in providing continual availability of a counsellor. While the NYUCI is being implemented in six states and in Israel, its effectiveness in the Hispanic community has not been tested. Thus, we proposed to test its effectiveness in this population conducting a community-based randomised trial called the Northern Manhattan Caregiver Intervention Project (NOCIP). We hypothesised that the NYUCI would be associated with greater reduction in depressive symptoms and burden compared with usual care after 6 months of the intervention.
NOCIP as a comparative effectiveness research project
NOCIP is a comparative effectiveness research (CER) project conducted under the auspices of the Northern Manhattan Center of Excellence in Comparative Effectiveness Research to Eliminate Disparities (NOCERED) funded by the National Institute of Minority Health and Health Disparities (NIMHD; 3P60MD000206-08S1) in the USA. CER32 can be defined as the direct comparison of existing interventions with proven efficacy to determine which work best for whom and which pose the greatest benefits and harms. Efficacy is the effect of an intervention under ideal conditions. Effectiveness is the effect of an intervention with proven efficacy in real-world settings. We chose to conduct a CER project in mental health of caregivers of persons with dementia because this is one of the priority areas for CER from the Institute of Medicine (interventions for caregivers of persons with dementia), and it also addresses two priority conditions for the Agency for Health Care Research and Quality (AHRQ): Dementia and Depression. We chose to test a counselling intervention in Hispanic caregivers because Hispanics have a greater burden of dementia compared with NHW, and because caregiving in Hispanics has particular characteristics, as explained before. We chose to test the effectiveness of the NYUCI because this is one of the most widely disseminated interventions for caregivers of persons with dementia. In addition, the NYUCI focuses on families, which may be particularly pertinent to Hispanic caregivers. NOCIP is an adaptation of the NYUCI to the realities of Hispanic caregivers in New York City.
Pilot data for 29 Hispanic spouse caregivers of persons with dementia in Northern Manhattan17 showed that all participants reported decreased sense of burden and depression and increased coping skills. However, community-based case management was deemed necessary in addition to the NYUCI in light of significant social and economic barriers that led to the caregivers feeling overwhelmed—this observation was used for justifying using the community health worker (CHW) intervention in this study as the control arm, and aiding the NYUCI arm. This pilot study also reported that Hispanic caregivers in Northern Manhattan perceive caring for ageing and ailing family members as a family affair, congruent with familism.33 Thus, embracing a family-centred perspective in all levels of assessment is key to effective communication and quality care for Hispanic caregivers. A family approach is a particular characteristic of the NYUCI that we hypothesised would be effective in the Hispanic community of New York City.
NOCIP is the first randomised trial testing the effectiveness of implementation of the NYUCI in an Urban Hispanic Community, in relative caregivers including spouses and non-spouses. NOCIP will provide important information about its potential benefits in this community.
Methods and analysis
The NOCIP (clinical trials.gov NCT01306695; PI: Luchsinger) is a RCT comparing an enhanced NYUCI intervention that includes a CHW case management component with a CHW case management intervention alone. The RCT is being conducted in a sample of 160 Hispanic family caregivers (80/arm) of persons with dementia mainly residing in the community of Northern Manhattan. The follow-up period is 6 months. Our study is approved by the Institutional Review Board of Columbia University Medical Center in New York City. Following consent, determination of eligibility and completion of baseline measures, the coordinator alerts the Data Coordinating Center (DCC) electronically either via encrypted email or data uploads to a secure server. Respondents are randomised to treatment or active placebo groups. The randomisation algorithm accommodates rolling enrolment, and the results are checked periodically for balance. All study personnel are fluent in English and Spanish. The study coordinator screens participants and gives eligibility information to the DCC at the Research Division of the Hebrew Home at Riverdale. The DCC provides the counsellor administering the NYUCI the identification numbers of participants randomised to the intervention. The study coordinator and the CHWs are blind to this randomisation process. Each participant is randomised to one of the two study CHWs, in order to maintain a balance of CHWs in the two study arms.
Participants
All study participants are caring for a family member (spouse, parent, sibling or other family member) with a clinical diagnosis of dementia. All people with dementia must be living at home when the caregiver enrols in the study.
The eligibility criteria for the study are the following:
Ethnicity: caregiver must be Hispanic.
Living arrangements: respondent is the spouse or is otherwise related to the care recipient and is a caregiver of the patient with dementia (although he/she does not have to live with the recipient).
Care receiver must have a diagnosis of dementia.
Care receiver must not have had a stroke with hemiparesis or any other motor impairment.
Care receiver is not confined to a wheelchair.
Care receiver does not suffer from Parkinson's disease.
Care receiver does not suffer from any other disorder that severely limits movement.
Caregiver does not have impaired speech.
Caregiver is physically able to provide care.
Caregiver does not have an exclusionary psychiatric diagnosis (depression with psychosis). Caregivers with clinical depression or other serious mental illness will be referred elsewhere for mental health treatment.
Respondent will be in the area for next 7 months (vacation of <4 weeks is ok).
The person with dementia or the caregiver has to be in contact with at least one relative or close friend living in the New York City metropolitan area.
Hearing is sufficient to allow for communication.
Rationale for eligibility criteria of participants
NOCIP was originally planned for Hispanic spouse caregivers of persons with dementia, as in the original efficacy trials of the NYUCI. However, we found early on in our recruitment effort that most Hispanic caregivers in New York City were not spouses. Some spouses were informal and included common-law partners, and divorced spouses taking care of their ex-spouses. In addition, caregivers included adult children, nephews/nieces and siblings. Thus, following the CER principle of testing interventions in the “real world”, we modified our inclusion criteria to include any relative caregiver with authorisation from the funding agency. At the time of submission of this article, of 93 caregivers enrolled in the trial, most were women (87.1%) and included 37 wives/partners (39.7%), 41 daughters (44.0%), 2 sisters (2.1%) and 1 niece (1%).
In addition to targeting all relative caregivers compared to only spouse caregivers, our project has other important differences compared to the original studies of the NYUCI. The original NYUCI targeted caregivers of spouses with AD. Our study targets caregivers of persons with dementia of any type as long as the patient does not have a significant motor deficit (eg, hemiparesis from a stroke). This makes sense because it is increasingly accepted that dementia is more heterogeneous than previously thought34 and that boundaries between dementia subtypes (eg, AD vs vascular dementia) are arbitrary. The rationale for excluding persons with motor deficits is that these deficits represent an additional burden not targeted by the NYUCI.
Recruitment
The sampling frame for recruitment is self-identified Hispanic caregivers of persons with dementia in New York City. Our recruitment methods include:
Promoting the study in the local memory disorders clinic.
Promoting the study among physicians taking care of elderly persons in the Ambulatory Care Network of New York Presbyterian Hospital.
Participation in health fairs and community talks.
Promotion at caregiver support groups and senior centres.
Mailing postcards promoting the study with assistance of a marketing company targeting households in Northern Manhattan with Hispanics aged 40 years and older.
Mailing households on mailing lists of organisations of dementia caregivers in New York City.
Consideration of recruitment strategies
We considered several recruitment strategies. The easiest way to recruit for this study and our preferred approach would be to identify persons with dementia from administrative datasets with inpatient and outpatient information, including ICD-9 codes for dementia and information on emergency contacts and next of kin. However, this approach was not approved by the hospital centre because of several concerns. First, neither the person with dementia nor the caregiver had given consent to access their administrative data for research purposes. Second, the persons with dementia would not have the capacity to provide such consent and could not be consulted. Third, there would be no assurance that the listed next of kin or other caregiver contacted was actually aware of the diagnosis of dementia; this situation could cause harm if the investigators contacted family members who were unaware of the diagnosis of dementia. Thus, we have resorted to the strategies described in the methods section, including recruitment in the community (particularly senior centres and caregiver programmes), through outreach in medical services, including general medicine, geriatric practices, psychiatry practices and memory disorders clinics and through targeted talks to caregivers groups.
Summary of interventions
Both the NYUCI and the CHW intervention are carried out by bilingual personnel. The NYUCI is carried out by a counsellor with a Masters in Social Work (MSW) degree who has experience in dementia and caregiving issues. The CHW have at least a 2-year college degree (eg, health education), and are trained at a community-based organisation named Alianza Dominicana, Inc, following a curriculum for CHWs that has been previously established. All study visits and those for the interventions are carried out in the participants’ homes or place of preference. In the case of the intervention arm, in which participants receive both the CHW intervention and NYUCI, these interventions are carried out independently. There is no communication between the NYUCI counsellor and the CHW, and their visits do not coincide. The NYUCI is an active counselling intervention that targets specific issues related to caregiving for persons with dementia. The CHW intervention targets general well-being and provides passive information about resources for caregivers of persons with dementia.
Overview of the NYU caregiver intervention
The first component consists of two individual and four family counselling sessions that include relatives suggested by the caregiver. The content of these sessions is determined by the needs of each caregiver and other participating family members (eg, learning techniques for management of troublesome patient behaviour, and promoting communication among family members). These sessions last between 1 and 1.5 h. The second component of the intervention is participation in a support group to provide the caregiver with continuous emotional support and education. The third component of the treatment is ‘ad hoc’ counselling—the continuous availability of counsellors to caregivers and families to help them deal with crises and with the changing nature and severity of their relatives’ symptoms over the course of the disease. The emergence of new psychiatric and behavioural problems of patients, which are generally more stressful than the need for assistance with activities of daily living or physical limitations, often precipitate ad hoc calls from caregivers. Ad hoc counselling makes it possible for caregivers and families to determine the amount of contact they have with the counsellors beyond the scheduled structured sessions.
The NYUCI is being administered by a bilingual (English, Spanish) counsellor with an MSW degree and has experience with dementia.
Overview of the CHW intervention
The CHW intervention consists of two visits in month 1, followed by monthly visits until month 6. The main role of the CHW is to provide access to existing education and referral resources about dementia and caregiving. In addition, the CHW assesses other health and social issues and provides information on existing resources in New York City. The CHW carries a smartphone with real-time access to email, text, the internet and telephone. CHWs also provide participants with their telephone number and email address for ad-hoc contacts.
The CHWs are based at Alianza Dominicana. In summary, the CHWs provide existing written information from the New York City chapter of the Alzheimer's Association, medical resources related to dementia at local medical centres and the community of Northern Manhattan, senior centres, and support groups. This information is given in the first visit in a manual written for this study and reinforced in all subsequent visits and on an ad hoc basis.
During the second through fourth home visit the CHW deals with barriers and goal setting. On the basis of patient needs and preferences the CHW assists the caregivers in developing an individualised plan towards maintaining their health and well-being. The CHW orients the participant in the principles of self-management35 36 and engages the participant in a problem-solving process to: Set priorities for immediate problem resolution; set personal goals; develop a plan to accomplish those goals, and review results and revise the plan as needed. While the focus of the research intervention is on dementia education, the CHWs address these issues from an overall health and general well-being approach. This is consistent with the CHW model that takes a patient centred approach and not a narrow disease-specific focus.
On the basis of the home assessment, CHW help with referrals to community-based resources that may be social or medically based, for example, accessing housing, public assistance, health insurance, immigration-related issues, day care (for children or elderly parents), services for domestic violence, etc, with the goal of eliminating immediate needs so the individual could make her/his health a priority. A strength of the community-based CHW model of service delivery is that through their training and exposure, CHWs are well aware of the existing programmes in the community and are able to play an active role in addressing these issues by serving as a point of contact for community-based resources.
Outcome variables
For the purpose of this translation of the NYUCI, the primary outcomes are depressive symptoms, measured with the Geriatric Depression Scale (GDS)37 and burden, measured with the Zarit Caregiver Burden Scale (ZCBS).38 Secondary outcomes include caregiver health, measured with the caregiver physical health form39 Revised Memory and Problem Behavior Checklist,40 the Stokes Social Network scale41 and an assessment of the severity of patient dementia with the Global Deterioration Scale.42 Additionally, several Patient Reported Outcomes Measurement and Information System (PROMIS) (NIHPROMIS.org) measures are included measuring the domains of physical functioning, depression and fatigue. These outcome variables are collected at baseline and 6-month visits by a bilingual study coordinator who is blind to group assignment.
Rationale for outcome variables
We considered several measures of depression and stress as our primary outcomes. The selection of the GDS as the primary measure of depression was based on the use of this measure in the original study of the NYUCI, and findings from the literature related to differential item functioning (DIF) in measures of depression. Although there are few studies of DIF among Latino samples, our review43 of DIF in depression measures showed that many Center for Epidemiologic Studies Depression Scale (CES-D) items were biased for ethnically diverse groups. Thus, we are using the GDS, which although also containing items with DIF, have fewer such items, and less with somatic content. The latter have been found to be problematic with older individuals with comorbidities;44 such individuals will likely comprise a large part of the caregiver sample. Additionally, we are using the short-form depression measure from the National Institutes of Health PROMIS item bank45 46 as a secondary outcome. This measure has been found to be relatively DIF-free in the limited studies conducted. It has not been tested for DIF among Latino elderly, nonetheless, because of its primacy in future studies of depression, we will include it as an exploratory endpoint measure. The ZCBS was chosen because it has been shown to be a good measure of dementia caregiver burden and caregiver collapse.47 The ZCBS has been shown to improve in Hispanic communities in South Florida with the Resources for Enhancing Alzheimer's Caregiver Health (REACH), another intervention for dementia caregivers,48–50 and has also been used in studies of the NYUCI.22 Since an objective of the NYUCI and our CHW intervention is to alleviate caregiver burden we chose to include a measure of caregiver burden previously used in Hispanics as a co-primary outcome.
Statistical plan and data analysis
Sample size and power analysis
Assumptions
The sample size calculations are based on the number of subjects needed to provide adequate power to test the primary hypothesis related to group differences in depression and burden at 6-month follow-up. The primary power calculations assume separate analyses of burden and depression measures; however, the use of MANOVA (multivariate analysis of variance) to perform a simultaneous test was also considered because it is generally more powerful, and makes use of more information. In addition, although full information likelihood estimation procedures will be used for the primary analyses, thereby allowing us to include participants who do not complete the follow-up assessment (on an intent-to-treat (ITT) basis), the power calculations include scenarios in which there is loss to follow-up as large as 20%. Based on the trial data extant, heterogeneous variances are not expected; however, this possibility was considered.
Effect sizes (Cohen's d51) for depression and burden
Studies of caregivers have used different depression measures; for example, the REACH study48 used the CES-D,52 whereas the NYUCI study used the GDS.37. Both studies used the Zarit Burden Interview (ZBI).38 For the ZBI, we used the estimates from REACH because they included a sample of Hispanic caregivers; for the depression measure we used estimates from the GDS provided from the NYUCI study. Based on these studies, the following data were used for estimation: the baseline ZBI SDs in the NYU study were 9.46 and 10.86 in the treatment and enhanced care groups, respectively. In the Hispanic REACH sample, the estimates of the parameters are as follows:μT1 (treatment group mean and (SD)=16.9 (9.6); μT2(6-month follow-up)=4.9 (9.1)μC1 (control group)=17.4 (9.9); μC2=15.9 (9.9). The estimate used for α (reliability) was 0.85 and for r (correlation between times 1 and 2) between 0.50 and 0.70. The estimate used for σ (pooled SD) was ≈9.8. Because the SD for both the ZBI and the GDS in these studies was almost the same, we focused our power calculations on ZBI, realising that most apply equally to the GDS. A SAS macro was used for power calculations.
Power for endpoint analyses
Although the primary analyses proposed a full information mixed model approach, to be conservative, the power calculations were also examined based on a two-group comparison of endpoint means (differences in means), with possible attrition. It was assumed that because of randomisation that there would be no need for baseline adjustment. The following assumptions were made: σ=9.8, α=0.05, R=0.85 (reliability), g=2 (groups). Assuming power of 0.80, with 80 per group (160 total) we would be able to detect a moderate effect size (Cohen's d=0.48)—equivalent to about 4.71 points on the ZBI or GDS—about a 5-point endpoint mean difference between groups. Sample size requirements were also examined for the detection of other endpoint differences: 4.0, 4.5 points and 5 points. Sample sizes to detect this range of effects are: 111, 88 and 71, respectively. Also examined were different scenarios regarding correlations between baseline and 6-month follow-up outcome measures. The following formula from Fleiss (pp. 4–5)53 was modified to include different scenarios related to correlations between the two waves of data:
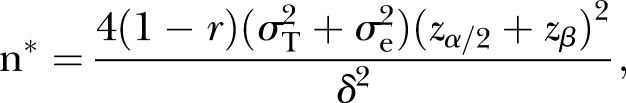 |
adjusting for unreliability: 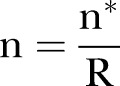 .
.
Assuming a sample size of 80 per group, and r (correlations between waves of data)=0.5, 0.6 and 0.7, the resulting estimates of effect sizes are δ=4.71, 4.21 and 3.65, thus demonstrating that a medium effect size (Cohen's d=0.37 to 0.48) could be detected with this sample size.
Power for longitudinal multivariate analyses
Assuming that the outcomes are correlated, power for MANCOVA was performed, taking into account possible baseline differences (using the observed means from the Hispanic REACH study) and adjusting for unreliability. We also modelled different correlations between the first and second waves of data.
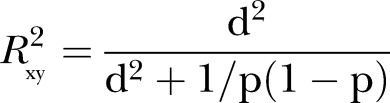 |
where 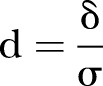 and p is a proportion of the combined populations in either of the populations (p=0.5 for equal size in the intervention and control groups) (Cohen, pp. 490–493), and
and p is a proportion of the combined populations in either of the populations (p=0.5 for equal size in the intervention and control groups) (Cohen, pp. 490–493), and
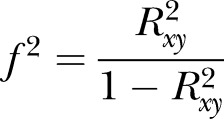 |
and 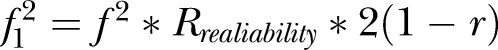 (adjusted for unreliability and r). The non-centrality parameter (λ) is
(adjusted for unreliability and r). The non-centrality parameter (λ) is 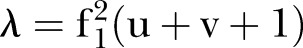 where u=ky (outcomes in MANOVA), and v=N−ky−1 (N=total sample size). The effect sizes were obtained in an iterative procedure, based on the assumptions shown in table 1.
where u=ky (outcomes in MANOVA), and v=N−ky−1 (N=total sample size). The effect sizes were obtained in an iterative procedure, based on the assumptions shown in table 1.
Table 1.
Assumptions for power calculations and effect sizes for MANOVA
| MANOVA assumptions: σ=9.8, α=0.05, R (reliability)=0.85; g=2 groups, 1-β=0.80), M=80/group, two outcomes | Point reduction in the intervention relative to control group (δ) |
| r (correlation between waves)=0.5 | 5.30 (Cohen d=0.54) |
| r=0.6 | 4.75 (Cohen d=0.48) |
| r=0.7 | 4.10 (Cohen d=0.42) |
MANOVA, multivariate analysis of variance.
Power for MANOVA was also examined under several scenarios regarding the non-centrality parameter. The resulting λ's are shown in table 2. The following assumptions were made: α=0.05, σ=9.8; reliability= 0.85.δ=5 point reduction in the intervention relative to the control group.
Table 2.
Power for multivariate analysis of variance examined under several scenarios regarding the non-centrality parameter
| (80/group) | r=0.5 | r=0.6 | r=0.7 |
|---|---|---|---|
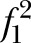 |
0.0553 | 0.0691 | 0.0922 |
| λ's | 8.85 | 11.06 | 14.75 |
Power for comparing rates of change over time in response between groups
Power to detect a difference in slopes (β1A−β1B) over the 6 months of the study was examined. The following formula provides an estimate of the required sample sizes54 55: m=(σ2(1−r)2(Zα/2+Zβ)2)/nsx2d2, where xj is time, measured as the duration between the first and jth occasion, j=0,1. The same assumptions as above were made. A smaller n (about 56 per group) was required. Although power is greater for evaluation of slopes over endpoint means, it is not recommended that sample sizes be less than 80, given that the power calculations in the REACH project also yielded requirements in the range of 80−100 per group.
In summary, across the methods (see table 3) Cohen's d ranged from 0.37 to 0.54 or between 4 and 5 points on the ZBI or GDS endpoint means—roughly equivalent to a 0.5 SD endpoint difference in means—or a moderate effect size. Conservatively, under the assumptions specified above, 80 subjects per group will provide power ≥0.80 to detect a 4−5 unit differential change in depression and burden, based on testing the time × group interaction, allowing for heterogeneous variances and serial correlations (figure 1). Even if the pooled variance is higher than assumed, medium effect sizes are still detectable. Thus, 80 subjects per arm will provide sufficient power to detect the hypothesised difference between the active control and the intervention arm of the study.
Table 3.
Summary of effect sizes for different approaches to power calculations (a,b)
| Method | Effect size (Δ/σ) |
|---|---|
| (a) Two-group endpoint differences | 0.48 |
| (b) Two-group endpoint differences, different r | 0.37–0.48 |
| (c) MANOVA (two groups, r=0.5, 0.6, 0.7) | 0.54, 0.48, 0.42 |
Endpoint differences under different assumptions about the correlation between baseline and endpoint mean values of the outcomes (r) and (c) MANOVA under different scenarios regarding r.
MANOVA, multivariate analysis of variance.
Figure 1.
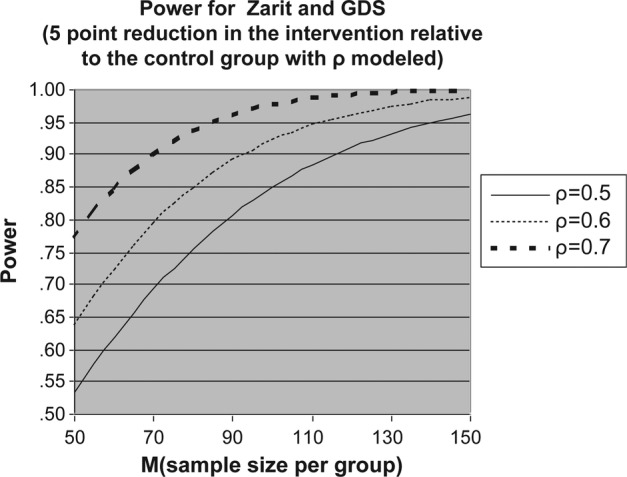
Power for examining change over time in the outcomes of the the Zarit Caregiver Burden Scale (ZCBS) and the Geriatric Depression Scale (GDS).
Analytic plan
Our approach to the analyses is guided by our own experience,56 and recent reviews of the relative advantages of constrained full information likelihood treatment of the outcome versus inclusion of the baseline value as a covariate in ANCOVA,57 and methods of estimation (generalised estimating equations (GEE) vs mixed random effects).58 The primary proposed analyses will use mixed random effects models, and a Full Information Maximum Likelihood (FIML) approach, with sensitivity analyses using GEE. The change from pretreatment to post-treatment values of continuous outcomes will be modelled as functions of baseline values, treatment and the interaction of baseline and treatment. Such an approach allows for possible group heterogeneity in residual variances and serial correlations that may require modelling. Based on prior analytic experience with the outcome variables, it is not expected that transformations will be necessary.
Prior to analyses, baseline values of all variables from each arm will be examined; however, no p values will be provided, and covariates are not proposed for inclusion in the main analyses of treatment effects. Depending on the severity of missing data, other modelling techniques may be used. Examination of baseline differences on key variables between completers and those lost-to-follow-up will be conducted to inform about the nature of the missing data. The ITT analyses performed using SAS PROC MIXED will permit all individuals with at least one observation to be included. Other methods of examining missing data (eg, propensity scores and multiple imputation) will be considered in sensitivity analyses.
Depending on the observed correlation between the dependent variables, MANOVA or MANCOVA (multivariate analysis of covariance) will be performed in sensitivity analyses. A significant interaction term for one of the groups would indicate that the effect of one of the treatments is different for ZBI and GDS; in that case, two treatment effects will be estimated for each outcome. If the interaction is not significant, a model with only main effects for the outcomes and treatment will be fit and the treatment effect (common for ZBI and GDS will be estimated from this model. In addition to significance testing, we will estimate the treatment effects with 95% CI. The general hypothesis is that, controlling for covariates (if needed), the vector of means will differ over time between groups. Or we can adjust each vector of means for prescore level, and test the hypothesis of equality of means for the groups using Wilks’ λ or Hotelling's T2. More powerful methods such as a risk score test 59 may be used, depending upon whether all endpoints are affected equally or not. Bartlett's test of sphericity will inform about the degree of intercorrelation among the outcome measures in order to determine suitability of the basic MANOVA model. Using collinearity diagnostics and examination of correlations, the final covariate set will be selected. It is anticipated that there will be: ky=2 non-redundant outcomes (depression and burden) kc=1 exogenous baseline covariate kx=1 dummy variable (NYU intervention). Depending on the results of the analyses of bias due to attrition or selection, other covariates may be included.
Possible attrition bias will be addressed using information from the baseline assessment. Completers and dropouts will be compared with respect to sociodemographics, baseline ZBI and GDS and other covariates. A logit model of attrition will be developed. If attrition is significantly related to one or more baseline characteristics, the predicted values from this model can be used as a covariate to adjust for differential attrition. Depending on the degree of bias, another approach is to perform propensity score analyses, in which the treatment groups are combined, and a logistic regression predicting original group membership from covariates performed. The resulting probabilities are then arrayed in quintiles, and the subjects within each quintile randomly assigned to new groups. The analyses will be re-run with the new group designations in order to determine if the effects were similar in the new analyses with groups equalised.
Under the assumption that the missing data are either Missing Completely at Random (MCAR) or Missing at Random (MAR), we will use the above-described maximum-likelihood approach to estimate treatment effects including the baseline data for these subjects in the analysis, in conjunction with the covariate to adjust for attrition bias (if necessary). Scales will be prorated for missing data, using individual imputation algorithms developed by the measurement statisticians at the DCC. Missing data are only replaced for those who are missing less than 50% of items.
Because the analysis is based on ITT, an attempt will be made to obtain post-treatment data from all participants randomised, regardless of level of attendance, thus minimising loss to 6-month follow-up. Because most programmes do not retain all participants nor do all receive the same ‘dose’ of the intervention, inclusion of participants who received only part of their targeted programme is more reflective of the real-world impact. Secondary analyses will be conducted to investigate the impact of differential participation, stratifying the participants in the treatment conditions based on their degree of participation and examining differences between strata on the outcome measures at follow-up. However, it is acknowledged that the sample sizes are small for such analyses.
Ethics and dissemination
This study is approved by the Institutional Review Board of Columbia University Medical Center (AAAI0022). The study is monitored by a three-member Data Safety and Monitoring Board (DSMB) that is convened twice a year. The DSMB is comprised of an expert in clinical trials of behavioural interventions, a neurologist with expertise in dementia and mental health, and a social worker with expertise in counselling interventions for persons with dementia. The DSMB is provided with up-to-date recruitment and adverse events data. The results of the study will be submitted for publication in a peer-reviewed journal. In addition, we will submit manuscripts on the characteristics of Hispanic caregivers in New York City once recruitment is completed. The funding agency will have no role in the content of these manuscripts.
Registration
This project is registered in the USA in the clinicaltrials.gov website (NCT01306695).
Supplementary Material
Acknowledgments
We would like to express our gratitude to the New York City Chapter of the Alzheimer's Association, the Washington Heights Inwood Council on Aging, New York Presbyterian Hospital and the Hebrew Home for the Aged for supporting the outreach and recruitment activities related to this project. We would also like to thank Gabriela Torres-Patino and Dante Tapiani for carrying out the project and collecting data cited in this article.
Footnotes
Contributors: JAL is the study principal investigator and was responsible for obtaining funding, designing the study, and drafting this manuscript; MaM is the study co-principal investigator and was responsible for co-designing the study, designing the New York University Caregiver Intervention (NYUCI) implementation, and drafting this manuscript; MiM was responsible for designing the community health worker (CHW) intervention; RL was responsible for developing bioinformatics strategies for participant recruitment. SS was responsible for aiding in the design of the study logistics; JK was responsible for sample size calculations and the statistical analysis plan; MR was responsible for the design of study logistics and questionnaires; JT is the leader of the data coordinating centre (DCC) and was responsible for the overall study design, the statistical plan, and drafting of this manuscript. All authors read and approved the final manuscript.
Funding: The primary sources for funding for this project are grants from NIMHD (National Institute of Minority Health and Health Disparities) (3P60MD000206, 3P60MD000206-08S1 and 3P60MD000206-09S2). Partial support for development of this project was provided by a Collaborative and Multidisciplinary Pilot Research Award (CaMPR) from the Irving Institute of Clinical Translational Research at Columbia University Medical Center, funded by a Clinical Translational Science Award (UL1 RR024156). The content is solely the responsibility of the authors and does necessarily represent the official views of the NIH.
Competing interests: MaM is the developer of the NYUCI and has received consulting fees for training providers. She is currently working on a Small Business Innovation Research grant to develop online training for the NYUCI. It is possible that MaM will benefit in the future from the distribution of NYUCI training materials. The other author(s) declare that they have no competing interests.
Ethics approval: Columbia University Medical Center Institutional Review Board.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1.Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). Washington, DC: American Psychiatric Association, 1994 [Google Scholar]
- 2.Cummings JL. Alzheimer's disease. N Engl J Med 2004;351:56–67 [DOI] [PubMed] [Google Scholar]
- 3.Thies W, Bleiler L. 2011 Alzheimer's disease facts and figures. Alzheimers Dement 2011;7:208–44 [DOI] [PubMed] [Google Scholar]
- 4.Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke—Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 2006;37:2220–41 [DOI] [PubMed] [Google Scholar]
- 5.Seshadri S, Wolf PA, Beiser A, et al. Lifetime risk of dementia and Alzheimer's disease. The impact of mortality on risk estimates in the Framingham Study. Neurology 1997;49:1498–504 [DOI] [PubMed] [Google Scholar]
- 6.Evans DA, Funkenstein HH, Albert MS, et al. Prevalence of Alzheimer's disease in a community population of older persons. Higher than previously reported. JAMA 1989;262:2551–6 [PubMed] [Google Scholar]
- 7.NIH Consensus Development Conference Statement on Preventing Alzheimer's Disease and Cognitive Decline. NIH Consensus and State-of-the-Art Statements 2010;27 [PubMed] [Google Scholar]
- 8.Lyketsos CG, Steele C, Baker L, et al. Major and minor depression in Alzheimer's disease: prevalence and impact. J Neuropsychiat Clin Neurosci 1997;9:556–61 [DOI] [PubMed] [Google Scholar]
- 9.Lyketsos CG, Sheppard JM, Steinberg M, et al. Neuropsychiatric disturbance in Alzheimer's disease clusters into three groups: the Cache County study. 2001:1043–53 [DOI] [PubMed] [Google Scholar]
- 10.Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA 2002;288:1475–83 [DOI] [PubMed] [Google Scholar]
- 11.Monin JK, Schulz R. Interpersonal effects of suffering in older adult caregiving relationships. Psychol Aging 2009;24:681–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA 2002;287:2090–7 [DOI] [PubMed] [Google Scholar]
- 13.Taylor DH, Jr, Ezell M, Kuchibhatla M, et al. Identifying trajectories of depressive symptoms for women caring for their husbands with dementia. J Am Geriatr Soc 2008;56:322–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Covinsky KE, Newcomer R, Fox P, et al. Patient and caregiver characteristics associated with depression in caregivers of patients with dementia. J Gen Intern Med 2003;18:1006–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Port CL, Zimmerman S, Williams CS, et al. Families filling the gap: comparing family involvement for assisted living and nursing home residents with dementia. Gerontologist 2005;45 Spec No 1:87–95 [DOI] [PubMed] [Google Scholar]
- 16.Schulz R, Belle SH, Czaja SJ, et al. Long-term care placement of dementia patients and caregiver health and well-being. JAMA 2004;292:961–7 [DOI] [PubMed] [Google Scholar]
- 17.Rosenthal-Gelman C. Learning from recruitment challenges: barriers to diagnosis, treatment, and research participation for Latinos with symptoms of Alzheimer's disease. J Gerontol Soc Work 2010;53:94–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noble JM, Manly JJ, Schupf N, et al. Type 2 diabetes and ethnic disparities in cognitive impairment. Ethn Dis 2012;22:83–44 [PMC free article] [PubMed] [Google Scholar]
- 19.Tang MX, Stern Y, Marder K, et al. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. J Am Med Assoc 1998;279:751–5 [DOI] [PubMed] [Google Scholar]
- 20.Humes KR, Jones NA, Ramirez RR. Overview of race and Hispanic origin: 2010. In: Bureau UC, ed. 2010 Census briefs. Washington, DC: U.S. Department of Commerce, 2011 [Google Scholar]
- 21.2011. Alzheimer's disease facts and figures. Alzheimers Dement 2011;7:208–44 [DOI] [PubMed] [Google Scholar]
- 22.Gaugler JE, Mittelman MS, Hepburn K, et al. Predictors of change in caregiver burden and depressive symptoms following nursing home admission. Psychol Aging 2009;24:385–96 doi:2009-08094-011 (pii) 10.1037/a0016052 (published Online First: Epub Date)| [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaugler JE, Roth DL, Haley WE, et al. Can counseling and support reduce burden and depressive symptoms in caregivers of people with Alzheimer's disease during the transition to institutionalization? Results from the New York University caregiver intervention study. J Am Geriatr Soc 2008;56:421–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mittelman MS, Brodaty H, Wallen AS, et al. A three-country randomized controlled trial of a psychosocial intervention for caregivers combined with pharmacological treatment for patients with Alzheimer disease: effects on caregiver depression. Am J Geriatr Psychiatry 2008;16:893–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mittelman MS, Roth DL, Clay OJ, et al. Preserving health of Alzheimer caregivers: impact of a spouse caregiver intervention. Am J Geriatr Psychiatry 2007;15:780–9 [DOI] [PubMed] [Google Scholar]
- 26.Drentea P, Clay OJ, Roth DL, et al. Predictors of improvement in social support: five-year effects of a structured intervention for caregivers of spouses with Alzheimer's disease. Soc Sci Med 2006;63:957–67 [DOI] [PubMed] [Google Scholar]
- 27.Mittelman MS, Haley WE, Clay OJ, et al. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology 2006;67:1592–9 [DOI] [PubMed] [Google Scholar]
- 28.Roth DL, Mittelman MS, Clay OJ, et al. Changes in social support as mediators of the impact of a psychosocial intervention for spouse caregivers of persons with Alzheimer's disease. Psychol Aging 2005;20:634–44 [DOI] [PubMed] [Google Scholar]
- 29.Mittelman MS, Roth DL, Haley WE, et al. Effects of a caregiver intervention on negative caregiver appraisals of behavior problems in patients with Alzheimer's disease: results of a randomized trial. J Gerontol B Psychol Sci Soc Sci 2004;59:P27–34 [DOI] [PubMed] [Google Scholar]
- 30.Mittelman MS, Roth DL, Coon DW, et al. Sustained benefit of supportive intervention for depressive symptoms in caregivers of patients with Alzheimer's disease. Am J Psychiatry 2004;161:850–6 [DOI] [PubMed] [Google Scholar]
- 31.Mittelman MS, Epstein C, Pierzchala A. Counseling the Alzheimer's caregiver: a resource for health care professionals. Chicago: AMA Press, 2003 [Google Scholar]
- 32.Sox HC, Greenfield S. Comparative effectiveness research: a report from the Institute of Medicine. Ann Intern Med 2009;151:203–5 [DOI] [PubMed] [Google Scholar]
- 33.Neary SR, Mahoney DF. Dementia caregiving: the experiences of Hispanic/Latino caregivers. J Transcult Nurs 2005;16:163–70 [DOI] [PubMed] [Google Scholar]
- 34.Savva GM, Wharton SB, Ince PG, et al. Age, neuropathology, and dementia. N Engl J Med 2009;360:2302–9 [DOI] [PubMed] [Google Scholar]
- 35.Gonzales V, Hernandez-Marin M, Lorig K, et al. Tormando control de su salud: Una guia para el manejo de las enfermiedades del corazon, diabetes, asma, bronquitis, enfisema y otros problemas cronicos. Boulder, CO: Bull Publishing Company, 2002 [Google Scholar]
- 36.Lorig KR, Sobel DS, Stewart AL, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Med Care 1999;37:5–14 [DOI] [PubMed] [Google Scholar]
- 37.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982;17:37–49 [DOI] [PubMed] [Google Scholar]
- 38.Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist 1980;20:649–55 [DOI] [PubMed] [Google Scholar]
- 39.Fitzgerald JF, Smith DM, Martin DK, et al. Replication of the multidimensionality of activities of daily living. J Gerontol 1993;48:S28–32 [DOI] [PubMed] [Google Scholar]
- 40.Teri L, Truax P, Logsdon R, et al. Assessment of behavioral problems in dementia: the revised memory and behavior problems checklist. Psychol Aging 1992;7:622–31 [DOI] [PubMed] [Google Scholar]
- 41.Stokes JP. Toward an understanding of cohesion in personal change groups. Int J Group Psychother 1983;33:449–67 [DOI] [PubMed] [Google Scholar]
- 42.Reisberg B, Ferris SH, de Leon MJ, et al. The global deterioration scale for assessment of primary degenerative dementia. Am J Psychiatry 1982;139:1136–9 [DOI] [PubMed] [Google Scholar]
- 43.Teresi JA, Ramirez M, Lai JS, et al. Occurrences and sources of Differential Item Functioning (DIF) in patient-reported outcome measures: description of DIF methods, and review of measures of depression, quality of life and general health. Psychol Sci Q 2008;50:538. [PMC free article] [PubMed] [Google Scholar]
- 44.Grayson DA, Mackinnon A, Jorm AF, et al. Item bias in the Center for Epidemiologic Studies Depression Scale: effects of physical disorders and disability in an elderly community sample. J Gerontol B Psychol Sci Soc Sci 2000;55:P273–82 [DOI] [PubMed] [Google Scholar]
- 45.Reeve BB. Special issues for building computerized-adaptive tests for measuring patient-reported outcomes: the National Institute of Health's investment in new technology. Med Care 2006;44(11 Suppl 3):S198–204 [DOI] [PubMed] [Google Scholar]
- 46.Reeve BB, Hays RD, Bjorner JB, et al. Psychometric evaluation and calibration of health-related quality of life item banks: plans for the Patient-Reported Outcomes Measurement Information System (PROMIS). Med Care 2007;45(5 Suppl 1):S22–31 [DOI] [PubMed] [Google Scholar]
- 47.Gort AM, Mingot M, Gomez X, et al. Use of the Zarit scale for assessing caregiver burden and collapse in caregiving at home in dementias. Int J Geriatr Psychiatry 2007;22:957–62 [DOI] [PubMed] [Google Scholar]
- 48.Belle SH, Burgio L, Burns R, et al. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: a randomized, controlled trial. Ann Intern Med 2006;145:727–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wisniewski SR, Belle SH, Coon DW, et al. The Resources for Enhancing Alzheimer's Caregiver Health (REACH): project design and baseline characteristics. Psychol Aging 2003;18:375–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amanda FE, Louis DB, Jamie D. Enhancing Caregiver Health: Findings from the Resources for Enhancing Alzheimer's Caregiver Health II Intervention. J Am Geriatr Soc 2010;58(1):30–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York: Academic Press, 1977, 1988 [Google Scholar]
- 52.Radloff L. The CES-D scale: a self report depression scale for research in the general population. Appl Psychol Measur, 1977;1:385–401 [Google Scholar]
- 53.Fleiss JL. Design and analysis of clinical experiments. New York: John Wiley and Sons, 1986, 1999 [Google Scholar]
- 54.Liu G, Liang K-Y. Sample size calculations for studies with correlated observations. Biometrics 1997;53:937–47 [PubMed] [Google Scholar]
- 55.Diggle JP, Heagerty P, Liang KY, et al. Analysis of longitudinal data. New York: Oxford University Press, 2002 [Google Scholar]
- 56.Petkova E, Teresi J. Some statistical issues in the analyses of data from longitudinal studies of elderly chronic care populations. Psychosom Med 2002;64:531–47 [DOI] [PubMed] [Google Scholar]
- 57.Liu GF, Lu K, Mogg R, et al. Should baseline be a covariate or dependent variable in analyses of change from baseline in clinical trials? Stat Med 2009;28:2509–30 doi:10.1002/sim.3639 (published Online First: Epub Date)| [DOI] [PubMed] [Google Scholar]
- 58.Gardiner JC, Luo Z, Roman LA. Fixed effects, random effects and GEE: what are the differences? Stat Med 2009;28:221–39 [DOI] [PubMed] [Google Scholar]
- 59.Follman D. Multivariate tests for multiple endpoints in clinical trials. Stat Med 1995;14:1163–75 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


