Abstract
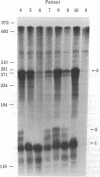
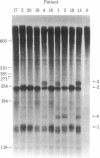
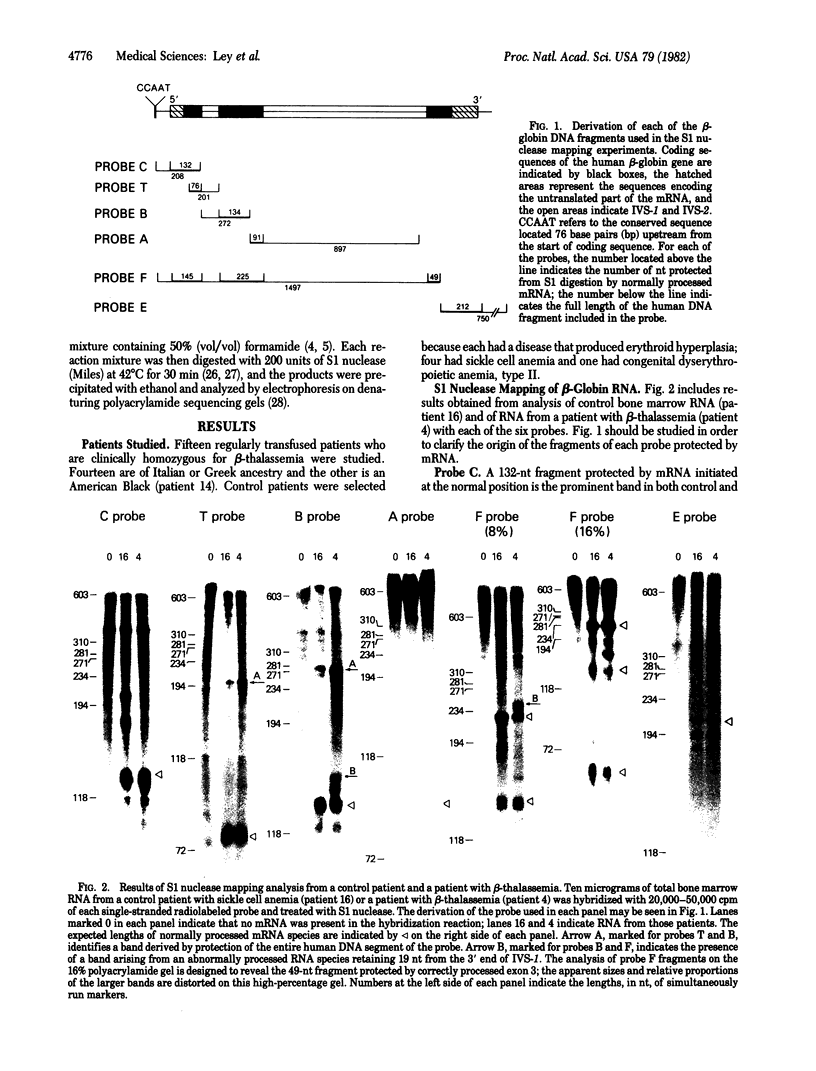
We have developed a method that permits rapid identification of the consequences of mutations that alter beta-globin RNA processing in erythroid cells. S1 nuclease mapping techniques were used to analyze total bone marrow RNA obtained fron 15 patients who are clinically homozygous for beta-thalassemia and from 5 patients with erythroid hyperplasia from other causes. This analysis was facilitated by the use of single-stranded uniformly labeled DNA probes of high specific activity that were prepared by using recombinant phage M13-beta-globin DNA templates. Two abnormalities of RNA processing were found to occur with high frequency in these patients. Nine thalassemic patients were found to have increased levels of an RNA species that retains all sequences transcribed from intervening sequence 1, implying the presence of mutations that decrease the correct splicing of this intron. Seven of 15 thalassemic patients were found to have an abnormally processed RNA species that retains 19 nucleotides transcribed from the 3' end of intron 1; this abnormality is caused by the G leads to A substitution in intron 1 that is known to create an alternative splice acceptor site.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird M., Driscoll C., Schreiner H., Sciarratta G. V., Sansone G., Niazi G., Ramirez F., Bank A. A nucleotide change at a splice junction in the human beta-globin gene is associated with beta 0-thalassemia. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4218–4221. doi: 10.1073/pnas.78.7.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank A., Mears J. G., Ramirez F. Disorders of human hemoglobin. Science. 1980 Feb 1;207(4430):486–493. doi: 10.1126/science.7352255. [DOI] [PubMed] [Google Scholar]
- Benz E. J., Jr, Scarpa A. L., Tonkonow B. L., Pearson H. A., Ritchey A. K. Posttranscriptional defects in beta-globin messenger RNA metabolism in beta-thalassemia: abnormal accumulation of beta-messenger RNA precursor sequences. J Clin Invest. 1981 Dec;68(6):1529–1538. doi: 10.1172/JCI110407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Busslinger M., Moschonas N., Flavell R. A. Beta + thalassemia: aberrant splicing results from a single point mutation in an intron. Cell. 1981 Dec;27(2 Pt 1):289–298. doi: 10.1016/0092-8674(81)90412-8. [DOI] [PubMed] [Google Scholar]
- Chang J. C., Kan Y. W. beta 0 thalassemia, a nonsense mutation in man. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2886–2889. doi: 10.1073/pnas.76.6.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumaki Y., Ghosh P. K., Benz E. J., Jr, Reddy V. B., Lebowitz P., Forget B. G., Weissman S. M. Abnormally spliced messenger RNA in erythroid cells from patients with beta+ thalassemia and monkey cells expressing a cloned beta+-thalassemic gene. Cell. 1982 Mar;28(3):585–593. doi: 10.1016/0092-8674(82)90213-6. [DOI] [PubMed] [Google Scholar]
- Gorski J., Fiori M., Mach B. A new nonsense mutation as the molecular basis for beta thalassaemia. J Mol Biol. 1982 Jan 25;154(3):537–540. doi: 10.1016/s0022-2836(82)80012-0. [DOI] [PubMed] [Google Scholar]
- Hamer D. H., Kaehler M., Leder P. A mouse globin gene promoter is functional in SV40. Cell. 1980 Oct;21(3):697–708. doi: 10.1016/0092-8674(80)90433-x. [DOI] [PubMed] [Google Scholar]
- Kantor J. A., Turner P. H., Nienhuis A. W. Beta Thalassemia: mutations which affect processing of the beta-Globin mRNA precursor. Cell. 1980 Aug;21(1):149–157. doi: 10.1016/0092-8674(80)90122-1. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Maquat L. E., Kinniburgh A. J., Beach L. R., Honig G. R., Lazerson J., Ershler W. B., Ross J. Processing of human beta-globin mRNA precursor to mRNA is defective in three patients with beta+-thalassemia. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4287–4291. doi: 10.1073/pnas.77.7.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquat L. E., Kinniburgh A. J., Rachmilewitz E. A., Ross J. Unstable beta-globin mRNA in mRNA-deficient beta o thalassemia. Cell. 1981 Dec;27(3 Pt 2):543–553. doi: 10.1016/0092-8674(81)90396-2. [DOI] [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschonas N., de Boer E., Grosveld F. G., Dahl H. H., Wright S., Shewmaker C. K., Flavell R. A. Structure and expression of a cloned beta o thalassaemic globin gene. Nucleic Acids Res. 1981 Sep 11;9(17):4391–4401. doi: 10.1093/nar/9.17.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhuis A. W., Canfield P. H., Anderson W. F. Hemoglobin messenger RNA from human bone marrow. Isolation and translation in homozygous and heterozygous beta-thalassemia. J Clin Invest. 1973 Jul;52(7):1735–1745. doi: 10.1172/JCI107355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhuis A. W., Turner P., Benz E. J., Jr Relative stability of alpha- and beta-globin messenger RNAs in homozygous beta+ thalassemia. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3960–3964. doi: 10.1073/pnas.74.9.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Goff S. C. Nonsense and frameshift mutations in beta 0-thalassemia detected in cloned beta-globin genes. J Biol Chem. 1981 Oct 10;256(19):9782–9784. [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr, Antonarakis S. E., Goff S. C., Boehm C. D., Sexton J. P., Waber P. G., Giardina P. J. Linkage of beta-thalassaemia mutations and beta-globin gene polymorphisms with DNA polymorphisms in human beta-globin gene cluster. Nature. 1982 Apr 15;296(5858):627–631. doi: 10.1038/296627a0. [DOI] [PubMed] [Google Scholar]
- Pergolizzi R., Spritz R. A., Spence S., Goossens M., Kan Y. W., Bank A. Two cloned beta thalassemia genes are associated with amber mutations at codon 39. Nucleic Acids Res. 1981 Dec 21;9(24):7065–7072. doi: 10.1093/nar/9.24.7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricca G. A., Taylor J. M., Kalinyak J. E. Simple rapid method for the synthesis of radioactively labeled cDNA hybridization probes utilizing bacteriophage M13mp7. Proc Natl Acad Sci U S A. 1982 Feb;79(3):724–728. doi: 10.1073/pnas.79.3.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Spritz R. A., Jagadeeswaran P., Choudary P. V., Biro P. A., Elder J. T., deRiel J. K., Manley J. L., Gefter M. L., Forget B. G., Weissman S. M. Base substitution in an intervening sequence of a beta+-thalassemic human globin gene. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2455–2459. doi: 10.1073/pnas.78.4.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trecartin R. F., Liebhaber S. A., Chang J. C., Lee K. Y., Kan Y. W., Furbetta M., Angius A., Cao A. beta zero thalassemia in Sardinia is caused by a nonsense mutation. J Clin Invest. 1981 Oct;68(4):1012–1017. doi: 10.1172/JCI110323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherall D. J., Clegg J. B. Recent developments in the molecular genetics of human hemoglobin. Cell. 1979 Mar;16(3):467–479. doi: 10.1016/0092-8674(79)90022-9. [DOI] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westaway D., Williamson R. An intron nucleotide sequence variant in a cloned beta +-thalassaemia globin gene. Nucleic Acids Res. 1981 Apr 24;9(8):1777–1788. doi: 10.1093/nar/9.8.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]