Abstract
Objective
To explore the relative association of admission blood glucose levels and antecedent diabetes on early and long-term survival in a contemporary UK population of patients with ST elevation myocardial infarction (STEMI) and non-STEMI (NSTEMI).
Design
Retrospective cohort study based on the Myocardial Ischaemia National Audit Project dataset.
Setting
Tertiary care centre.
Participants
4111 (20.3% known diabetes) consecutive patients admitted with acute myocardial infarction (58.3% STEMI) between October 2002 and September 2008.
Primary and secondary outcome measures
All-cause mortality at 30 days and 1 year. The relative association of admission blood glucose and of antecedent diabetes with mortality was assessed using multivariate Cox regression analysis. Furthermore, we compared these relationships in patients with STEMI to those with NSTEMI.
Results
By 30 days and 1 year, 409 (9.9%) and 677 (16.5%) of patients died. After adjusting for covariates, diabetes did not show independent association with mortality at any time point, in the entire cohort (HR 30 days 0.93 (95% CI 0.63 to 1.38); 1 year 1.00 (0.77 to 1.30)) or in subgroups of STEMI (HR 30 days 1.03 (0.65 to 1.64); 1 year 1.08 (0.77 to 1.51)) and NSTEMI (HR 30 days 0.62 (0.26 to 1.50); 1 year 0.87 (0.56 to 1.36)). In contrast, after adjusting for covariates, admission glucose showed robust and independent association with mortality in the entire cohort (HR: 30 days 1.07 (1.04 to 1.10); 1 year 1.05 (1.03 to 1.08)), and in the subgroup of STEMI (30 days 1.07 (1.03 to 1.10); 1 year 1.07 (1.04 to 1.10)), and NSTEMI (HR 30 days 1.07 (1.00 to 1.14); 1 year 1.02 (0.97 to 1.06)).
Conclusions
Admission glucose is strongly associated with mortality in all presentations of acute myocardial infarction (AMI), irrespective of established diabetes diagnosis. The increased risk is maintained up to 1 year. Future studies are required to assess the impact of active management of elevated blood glucose in improving mortality in individuals admitted with AMI.
Article Summary.
Article focus
Robust associations are seen for both measures of glycaemia—the diagnosis of diabetes, and elevated blood glucose levels on admission, with poor outcomes in patients with ST elevation myocardial infarction (STEMI).
We explored the less known, relative association of admission blood glucose levels and antecedent diabetes on early and long-term survival in a contemporary UK population of patients with STEMI and non-STEMI (NSTEMI).
Key messages
In patients with both STEMI as well as NSTEMI, admission glucose is more strongly associated with mortality than is antecedent diabetes diagnosis.
The increased risk associated with admission glucose is evident during the index admission, at 30 days, 1 year and beyond and is apparent in those surviving to discharge.
Conversely, after multivariate adjustment for covariates, including admission glucose is not associated with mortality.
Strengths and limitations of this study
This is a study of a large cohort of patients with both STEMI and NSTEMI managed in contemporary clinical practice in a tertiary care centre.
A statistically robust association was seen for admission glucose with both short-term and long-term mortality after adjusting for many important confounders.
Our data lack information on glucose-lowering intervention, patients with undiagnosed diabetes and other potentially relevant variables which were not considered in the analysis.
Introduction
For patients with acute myocardial infarction (AMI) the risk of adverse outcome is increased by the concomitant diagnosis of diabetes mellitus (diabetes).1 2 In addition, elevated blood glucose concentration, a common finding at admission in patients with AMI, is also associated with increased risk of adverse outcome, irrespective of prior diabetes.1–8 In some studies4 9 the association between admission blood glucose concentration and adverse outcome was more powerful in patients without, compared to those with, prior diabetes. Indeed, we previously reported more powerful association with 30-day and 1-year mortality after ST elevation myocardial infarction (STEMI) for admission blood glucose concentration, compared to the diagnosis of diabetes.9
While a causal relationship is unproven, there are numerous potential pathophysiological mechanisms by which hyperglycaemia may impart toxicity during myocardial ischaemia.10 11 Indeed, observational data suggest that elevated blood glucose may contribute directly to adverse outcome after AMI. Prognosis is worse for patients in whom hyperglycaemia persists in the 24–48 h after AMI compared to those in whom blood glucose normalises.12 13 In patients without prior diabetes, insulin-based treatment of hyperglycaemia after AMI is associated with improved prognosis.14 15 Further, in randomised, controlled trials (RCTs) of intensive, insulin-based blood glucose management during admission with AMI, survival benefit was evident only when intervention effectively lowered blood glucose concentration.16 17
While the relationship between blood glucose concentration and outcome after AMI has largely been described in patients with STEMI, the majority of acute coronary syndromes in contemporary practice are non-ST elevation AMI (NSTEMI). The aim of the current analysis was to compare the relative strength of association with 30-day and 1-year mortality of antecedent diabetes diagnosis and admission blood glucose concentration in patients with STEMI and with NSTEMI, and in those with and without a history of diabetes, in a multiethnic population. We also assessed the relevance of blood glucose concentration, recorded soon after admission to hospital with AMI, to mortality in patients surviving to discharge.
Methods
Data were from consecutive admissions between 1 October 2002 and 30 September 2008, to the two coronary care units of a large teaching hospital serving the population of Leicestershire, UK (approximately 946 000 residents in 2004). For all patients, as part of the hospital's mandatory commitment to the Myocardial Ischaemia National Audit Project (MINAP),18 we record clinical and demographic data including information on diagnosis (STEMI/NSTEMI), ECG site of infarct, medical history, coronary heart disease risk factors and prescribed medication. Data are record-linked to mortality information19 and include self-reported coding for ethnicity, for which local coverage is thorough. Approximately 10% of the local population are of South Asian ethnic origin, over twice the UK national average.
Patients were categorised as having a diagnosis of diabetes if this was self-reported by the patient, or on the basis of medication prescribed prior to admission. All patients with AMI routinely underwent blood glucose measurement, in most cases within first 12 h after admission with their blood samples assayed in the hospital laboratory. We used such first-recorded admission glucose levels for this analysis. All diagnoses of AMI were verified prior to submission to the national MINAP database; the diagnosis of AMI was made according to the joint ESC/ACCF/AHA/WHF definition.20 Patients were categorised as STEMI or NSTEMI, according to the final discharge diagnosis recorded in the MINAP database. For patients with multiple AMI admissions during the study period, we considered only the first event. The number of cases admitted with AMI during the study period determined the sample size.
Survival was measured from the date of first admission to the date of death or of censoring at 30 September 2009. Mortality data are supplied to the hospital on a monthly basis via the UK Office for National Statistics. Follow-up data on mortality were available for all the patients. The predefined primary outcome measure was 30-day, and 1-year, all-cause mortality.
The study was approved by the local research ethics committee. The data used in this analysis were gathered during routine care and as part of the MINAP18 mandatory requirement for all acute hospitals in England and Wales to collect data pertaining to admission with AMI.
Statistical analysis
Baseline characteristics were compared between groups using independent two-sample t tests for continuous variables and χ2 tests for categorical variables. Mortality at 30 days and at 1 year, in the entire cohort, and in those patients surviving to discharge, was calculated.
We calculated mortality proportions for patients admitted from 1 October 2002 to 30 September 2008 with follow-up censored at 30 September 2009. Survival probabilities were calculated using Kaplan-Meier (KM) analyses and patient groups compared using survival analysis log rank test. Relative risk of mortality, as a function of clinical variables, was examined using Cox proportional hazards techniques. We initially assessed the unadjusted, univariate association with outcome for admission blood glucose and for diabetes, and for other potentially relevant clinical and demographic variables (age, sex, ethnicity (White European, South Asian), smoking, type of AMI (STEMI, NSTEMI), prior history (hypertension, any coronary artery disease, cerebrovascular or peripheral vascular disease), admission systolic blood pressure and heart rate, estimated glomerular filtration rate (eGFR), coronary revascularisation during index admission, preadmission and discharge drug therapy (antiplatelet, β-blocker, statin, ACE inhibitor/angiotensin receptor blocker) and index loop diuretic use). An interaction term representing calendar year of admission was included to adjust for potential temporal changes in the management of acute coronary artery disease.
Demographic and clinical covariates with univariate association (p<0.10) with mortality at 30 days or 1 year were entered into multivariate models (Cox proportional hazards). All quantitative variables were entered as continues variables into the model. Patients with missing data (table 1) were not excluded but their values were set as missing. Statistical significance for all comparisons was set at p<0.05 (two-sided). Data are presented as HR and 95% CI. We used fractional polynominals to model admission glucose to account for any non-linearity and assessed its independent association with mortality in subgroups with and without diabetes. Analyses were carried out using SPSS V.18.
Table 1.
Baseline characteristics at admission stratified by diabetes status
| All, n=4111 | Known DM, n=835 (20.3%) | Not known DM, n=3276 (79.7%) | p Value* | Missing value (%) | |
|---|---|---|---|---|---|
| Demography | |||||
| Age (years) | 66.4 (13.3) | 68.6 (11.8) | 65.8 (13.6) | <0.005 | 0.0 |
| Women (%) | 1224 (29.8) | 276 (33.1) | 948 (28.9) | 0.022 | 0.0 |
| Ethnicity (%) | |||||
| White European | 3381 (82.2%) | 545 (16.1) | 2836 (86.6) | <0.005 | 0.0 |
| South Asian | 730 (17.8%) | 290 (39.7%) | 440 (60.3%) | 0.0 | |
| Medical history (%) | |||||
| Hypertension | 2048 (50.3) | 584 (70.0) | 1464 (45.0) | <0.005 | 1.0 |
| Current/ex-smoker | 1366 (35.7) | 282 (36.8) | 1084 (35.5) | 0.527 | 7.1 |
| Coronary heart disease† | 491 (12.1) | 149 (17.9) | 342 (10.6) | <0.005 | 0.9 |
| CVA | 254 (6.3) | 86 (10.3) | 168 (5.2) | <0.005 | 1.2 |
| PVD | 154 (3.8) | 42 (5.0) | 112 (3.5) | 0.041 | 1.2 |
| Heart failure | 190 (4.7) | 76 (9.1) | 114 (3.5) | <0.005 | 1.2 |
| Type of infarction (%) | |||||
| STEMI | 2397 (58.3) | 417 (49.9) | 1980 (60.4) | <0.005 | 0.0 |
| NSTEMI | 1714 (41.7) | 418 (50.1) | 1296 (39.6) | ||
| Physical examination | |||||
| Heart rate (beats/min) | 81.1 (24.3) | 85.5 (25.3) | 80.0 (24.0) | <0.005 | 1.5 |
| SBP (mm Hg) | 136.5 (28.4) | 137.7 (30.7) | 136.2 (27.8) | 0.202 | 1.0 |
| Biochemical data | |||||
| Peak CK (IU/l, normal range <200) | 1113.5 (1810.4) | 939.9 (1279.3) | 1156.4 (1917) | <0.005 | 7.6 |
| Creatinine (µmol/l) | 116.4 (63.8) | 128.8 (76.1) | 113.1 (59.8) | <0.005 | 16.8 |
| eGFR (ml/min) | 63.0 (22.2) | 57.7 (23.6) | 64.4 (21.7) | <0.005 | 16.6 |
| Total cholesterol (mmol/l) | 5.1 (1.3) | 4.4 (1.2) | 5.2 (1.3) | <0.005 | 16.6 |
| Haemoglobin (g/l) | 13.7 (1.9) | 13.0 (1.9) | 13.9 (1.8) | <0.005 | 66.6 |
| Plasma glucose (mmol/l) | 8.8 (4.2) | 12.0 (5.5) | 7.9 (3.3) | <0.005 | 14.9 |
| Therapies (%) | |||||
| Prior to index admission | |||||
| Aspirin | 2671 (65.0) | 622 (74.5) | 2049 (62.5) | <0.005 | 0.0 |
| β-Blocker | 990 (25.6) | 265 (33.2) | 725 (23.6) | <0.005 | 6.0 |
| ACE inhibitor or ARB | 1097 (28.3) | 407 (51.0) | 690 (22.5) | <0.005 | 5.8 |
| Statins | 1083 (28.0) | 389 (48.7) | 694 (22.6) | <0.005 | 5.8 |
| In-hospital | |||||
| Reperfusion therapy‡ | 2414 (58.7) | 419 (50.2) | 1995 (60.9) | <0.005 | 0.0 |
| Loop diuretics | 1502 (37.4) | 436 (52.7) | 1066 (33.4) | <0.005 | 2.3 |
| At discharge | |||||
| Aspirin | 2701 (68.1) | 529 (65.3) | 2172 (68.8) | 0.057 | 3.5 |
| β-Blocker | 2513 (63.3) | 483 (59.6) | 2030 (64.3) | 0.013 | 3.5 |
| ACE inhibitor or ARB | 2493 (62.9) | 495 (61.0) | 1998 (63.4) | 0.222 | 3.6 |
| Statin | 2704 (67.7) | 537 (65.6) | 2167 (68.2) | 0.167 | 2.8 |
All values are mean (SD) or number (%).
*Known diabetes versus not-known diabetes.
†Any of angina/myocardial infarction/percutaneous intervention (PCI)/coronary artery bypass grafting (CABG).
‡Thrombolysis or coronary intervention (PCI or CABG) or both.
ARB, angiotensin receptor blocker; CK, creatinine kinase; CVA, cerebrovascular accidents; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate calculated using the MDRD formula; MDRD, modification of diet in renal disease; NSTEMI, non-ST elevation myocardial infarction; PVD, peripheral vascular disease; STEMI, ST elevation myocardial infarction; SBP, systolic blood pressure.
Results
The study population was the 4111 patients admitted between 1 October 2002 and 30 September 2008 with discharge diagnosis of AMI (STEMI 2397, 58.3%) and for whom a minimum of 365 days follow-up was available from the date of admission. For this cohort, median follow-up was 912 (range 0–2556) days; for 3792 (92.2%) patients surviving to discharge from the index admission, median follow-up was 1031 (range 1–2556) days.
Demographic details of the study population are presented in table 1. Prior diabetes was recorded in 835 (20.3%) patients: compared to those without, patients with antecedent diabetes were on average older (68.6 vs 65.8 years, p<0.005), more likely to be female (33.9% vs 28.9%, p=0.022) and to have prior cardiovascular comorbidities. Presentation with NSTEMI was more prevalent in cases with (50.1%), compared to those without (39.6%), prior diabetes (p<0.005). Mean plasma glucose was higher in patients with diabetes (12.0±5.5 mmol/l) compared to those without (7.9±3.3 mmol/l) (p<0.005). Mean peak creatinine kinase was lower in patients with diabetes.
During the index admission administration of loop diuretic was more frequent (52.7% vs 33.4%, p<0.005) and, for patients with STEMI, coronary reperfusion therapy less frequent (50.2% vs 60.9%,<0.005), in patients with diabetes. Other than for slightly less use of β-blockers and aspirin in patients with diabetes, patterns of prescription of secondary prevention therapies at discharge were similar in the two groups.
Mortality—univariate analysis
Deaths during hospitalisation, over 30 days, 1 year and the entire period of follow-up numbered 319 (7.8%), 409 (9.9%), 677 (16.5%) and 1041 (25.3%), respectively. Age, female sex, higher admission heart rate, higher eGFR, lower systolic blood pressure and presentation with STEMI (compared to NSTEMI), as well as prior smoking and hypertension, each showed univariate association with mortality risk over all time periods (table 2). Loop diuretic was associated with a 3–4 fold increase in mortality during follow-up. Survival improved over the period of observation.
Table 2.
Univariate association of clinical variables with 30-day, 1-year and total mortality in the entire cohort
| N=4111 | Mortality, N (%) |
||
|---|---|---|---|
| 30 days | 1 year | All (median 912 days) | |
| 409 (9.95) | 677 (16.47) | 1041 (25.32) | |
| Admission demographic variable | |||
| Gender (female vs male) | 0.535 (0.439 to 0.650) | 0.515 (0.443 to 0.600) | 0.554 (0.490 to 0.627) |
| Age (year) | 1.068 (1.059 to 1.078) | 1.077 (1.069 to 1.084) | 1.084 (1.077 to 1.090) |
| SBP (mm Hg) | 0.979 (0.976 to 0.983) | 0.987 (0.984 to 0.990) | 0.992 (0.990 to 0.994) |
| Heart rate (beat/min) | 1.010 (1.006 to 1.013) | 1.012 (1.009 to 1.014) | 1.012 (1.010 to 1.014) |
| Total cholesterol (mmol/l) | 0.732 (0.666 to 0.806) | 0.765 (0.712 to 0.821) | 0.744 (0.703 to 0.788) |
| Admission plasma glucose (mmol/l) | 1.072 (1.052 to 1.084) | 1.065 (1.055 to 1.076) | 1.059 (1.050 to 1.068) |
| eGFR (ml/min) | 0.956 (0.951 to 0.961) | 0.955 (0.951 to 0.959) | 0.959 (0.956 to 0.962) |
| NSTEMI vs STEMI | 0.504 (0.405 to 0.627) | 0.736 (0.629 to 0.862) | 0.939 (0.830 to 1.063) |
| Year of admission | |||
| October 2002–December 2003 | 1 | 1 | 1 |
| 2004 | 0.909 (0.688 to 1.200) | 0.846 (0.681 to 1.052) | 0.919 (0.780 to 1.082) |
| 2005 | 0.591 (0.402 to 0.870) | 0.652 (0.491 to 0.865) | 0.702 (0.564 to 0.873) |
| 2006 | 0.830 (0.592 to 1.164) | 0.696 (0.529 to 0.917) | 0.716 (0.572 to 0.897) |
| 2007 | 0.759 (0.570 to 1.010) | 0.678 (0.541 to 0.849) | 0.679 (0.558 to 0.826) |
| 2008 | 0.485 (0.338 to 0.696) | 0.551 (0.424 to 0.716) | 0.531 (0.415 to 0.680) |
| Test for linear trend (p value) | <0.001 | <0.001 | <0.001 |
| Ethnicity (South Asian vs White European) | 1.013 (0.786 to 1.304) | 0.909 (0.741 to 1.114) | 0.856 (0.725 to 1.012) |
| Medical history (yes vs no) | |||
| Smoking | 1.016 (0.819 to 1.259) | 1.049 (0.891 to 1.235) | 1.160 (1.019 to 1.320) |
| Prior diabetes | 1.400 (1.121 to 1.750) | 1.576 (1.331 to 1.865) | 1.655 (1.445 to 1.896) |
| Prior coronary heart disease* | 0.862 (0.628 to 1.182) | 0.998 (0.791 to 1.258) | 1.113 (0.931 to 1.330) |
| Prior hypertension | 1.286 (1.056 to 1.567) | 1.437 (1.232 to 1.676) | 1.472 (1.300 to 1.666) |
| Preadmission medication (yes vs no) | |||
| Aspirin | 0.746 (0.613 to 0.909) | 0.869 (0.744 to 1.015) | 0.913 (0.804 to 1.036) |
| β-Blocker | 1.385 (1.116 to 1.719) | 1.577 (1.338 to 1.859) | 1.489 (1.301 to 1.703) |
| Statin | 0.994 (0.795 to 1.245) | 1.129 (0.953 to 1.338) | 1.194 (1.041 to 1.370) |
| ACE inhibitor or ARB | 1.242 (1.002 to 1.540) | 1.467 (1.247 to 1.726) | 1.621 (1.423 to 1.847) |
| Admission treatment (yes vs no) | |||
| Initial reperfusion | 0.616 (0.507 to 0.749) | 0.540 (0.464 to 0.629) | 0.466 (0.411 to 0.527) |
| Loop diuretic | 3.457 (2.807 to 4.256) | 4.348 (3.681 to 5.136) | 4.052 (3.556 to 4.618) |
| Discharge medication (yes vs no) | |||
| Aspirin | 0.043 (0.029 to 0.062) | 0.227 (0.192 to 0.269) | 0.439 (0.386 to 0.499) |
| β-Blocker | 0.038 (0.025 to 0.058) | 0.237 (0.199 to 0.282) | 0.406 (0.357 to 0.461) |
| Statin | 0.043 (0.029 to 0.062) | 0.196 (0.165 to 0.233) | 0.344 (0.303 to 0.390) |
| ACE inhibitor or ARB | 0.047 (0.031 to 0.700) | 0.236 (0.198 to 0.281) | 0.469 (0.412 to 0.533) |
*Any of angina/myocardial infarction/percutaneous intervention (PCI)/coronary artery bypass grafting (CABG).
Data are HR (95% CI).
ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate calculated using the MDRD formula; MDRD, modification of diet in renal disease; NSTEMI, non-ST elevation myocardial infarction; SBP, systolic blood pressure; STEMI, ST elevation myocardial infarction.
Prior diabetes showed strong univariate association with mortality risk over all time periods: HR 30 days 1.40 (1.12 to 1.75); 1 year 1.58 (1.33 to 1.86); all follow-up 1.66 (1.44 to 1.90; table 2). The strength of association between glucose and mortality was consistent at 30 day and at 1 year, each mmol/l increase in admission glucose concentration being associated with a 6–7% increase in hazard of mortality over all time periods.
Postdischarge mortality
In those surviving to discharge (N=3792), 106 (2.8%), 363 (9.6%) and 726 (19.1%) died by 30-day, 1-year and over all follow-up (see online supplementary table S2A). Univariate associations with mortality were similar to those in the entire population. Prior diabetes showed univariate association with increased risk of death at all times, although this was not statistically significant at 30 days (HR 1.36, (0.87 to 2.12)). For admission glucose, the strength of association with postdischarge mortality was very similar to that in the entire cohort, with 5–7% increase risk per mmol/l increase in glucose (see online supplementary table S2A).
Mortality—multivariate analysis
Table 3 shows the results of multivariate analysis. Age, lower admission systolic blood pressure and higher heart rate, lower eGFR, prescription of loop diuretic and STEMI (compared to NSTEMI) each retained independent association with mortality, as did prescription of individual discharge medications. After covariate adjustment, diabetes did not retain independent association with mortality at any time. In contrast, adjustment for covariates had little impact on the risk of mortality associated with admission glucose concentration.
Table 3.
Multivariate association of clinical variables with 30-day, 1-year and total mortality in the entire cohort
| N=4111 | Mortality, N (%) |
||
|---|---|---|---|
| 30 days | 1 year | All (median 912 days) | |
| 409 (9.95) | 677 (16.5) | 1041 (25.3) | |
| Admission demographics | |||
| Gender (female vs male) | 1.268 (0.885 to 1.819) | 1.094 (0.865 to 1.383) | 1.114 (0.931 to 1.332) |
| Age (year) | 1.059 (1.040 to 1.078) | 1.062 (1.048 to 1.075) | 1.073 (1.062 to 1.083) |
| SBP (mm Hg) | 0.987 (0.981 to 0.992) | 0.991 (0.987 to 0.995) | 0.993 (0.990 to 0.996) |
| Heart rate (beat/min) | 1.007 (1.001 to 1.013) | 1.006 (1.002 to 1.010) | 1.007 (1.005 to 1.010) |
| Admission plasma glucose (mmol/l) | 1.072 (1.042 to 1.104) | 1.059 (1.037 to 1.081) | 1.053 (1.036 to 1.071) |
| eGFR (ml/min) | 0.987 (0.978 to 0.996) | 0.983 (0.977 to 0.990) | 0.988 (0.983 to 0.993) |
| NSTEMI versus STEMI | 0.411 (0.282 to 0.597) | 0.558 (0.443 to 0.704) | 0.700 (0.587 to 0.834) |
| Ethnicity (South Asian vs White European) | 1.355 (0.893 to 2.057) | 1.155 (0.851 to 1.568) | 0.996 (0.779 to 1.273) |
| Medical History (yes vs no) | |||
| Smoking | 1.125 (0.788 to 1.607) | 0.953 (0.749 to 1.213) | 0.942 (0.786 to 1.130) |
| Prior diabetes | 0.934 (0.631 to 1.382) | 1.001 (0.770 to 1.300) | 1.134 (0.927 to 1.386) |
| Prior coronary heart disease* | 0.717 (0.402 to 1.278) | 0.898 (0.632 to 1.277) | 1.111 (0.864 to 1.428) |
| Prior hypertension | 1.291 (0.903 to 1.846) | 1.155 (0.913 to 1.461) | 1.133 (0.949 to 1.353) |
| Preadmission medication (yes vs no) | |||
| Aspirin | 0.944 (0.667 to 1.335) | 0.989 (0.781 to 1.252) | 1.010 (0.842 to 1.213) |
| β-Blocker | 1.288 (0.898 to 1.849) | 1.363 (1.067 to 1.742) | 1.173 (0.970 to 1.418) |
| Statin | 0.863 (0.579 to 1.286) | 0.877 (0.668 to 1.150) | 0.918 (0.743 to 1.135) |
| ACE inhibitor or ARB | 0.719 (0.497 to 1.042) | 0.932 (0.728 to 1.194) | 1.017 (0.840 to 1.232) |
| Admission treatment (yes vs no) | |||
| Loop diuretic | 1.416 (0.993 to 2.019) | 1.703 (1.322 to 2.195) | 1.532 (1.268 to 1.851) |
| Discharge medication (yes vs no) | |||
| Aspirin | 0.297 (0.157 to 0.562) | 0.656 (0.479 to 0.897) | 0.861 (0.676 to 1.097) |
| β-Blocker | 0.257 (0.133 to 0.494) | 0.564 (0.423 to 0.753) | 0.671 (0.544 to 0.828) |
| Statin | 0.628 (0.295 to 1.339) | 0.683 (0.484 to 0.963) | 0.629 (0.490 to 0.808) |
| ACE inhibitor or ARB | 0.470 (0.229 to 0.968) | 0.610 (0.443 to 0.839) | 0.850 (0.668 to 1.081) |
*Any of angina/myocardial Infarction/percutaneous Intervention (PCI)/coronary artery bypass grafting (CABG).
Data are HR (95% CI)
ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate calculated using the MDRD formula; MDRD, modification of diet in renal disease; NSTEMI, non-ST elevation myocardial infarction; SBP, systolic blood pressure; STEMI, ST elevation myocardial infarction.
Postdischarge mortality
For patients surviving to discharge, associations between clinical variables and the risk of mortality were similar to those seen in the entire cohort (see online supplementary table S3A). While there was no association between prior diabetes and risk of mortality at any time (HR 30 days 0.64 (0.31 to 1.300); 1 year 0.91 (0.66 to 1.26); all follow-up 1.08 (0.86 to 1.36)), blood glucose retained powerful association with the primary endpoint. This was evident at 30 days (HR per mmol/l 1.10, 95% CI 1.05 to 1.15), 1 year (1.05, 1.02 to 1.08) and over all follow-up (1.04, 1.02 to 1.06)).
Admission glucose concentration: influence on mortality in patients with or without diabetes
We repeated multivariate analysis including a term for interaction between diabetes diagnosis and admission glucose concentration. While numerically greater in individuals without diabetes (figure 1), there was no conventional statistically significant difference in the association between mortality and admission blood glucose for patients with and without diabetes (30 days HR 1.00 (95% CI 0.97 to 1.03, p=0.95; 1 year 0.99, (0.97 to 1.02), p=0.66; entire follow-up 0.99, (0.97 to 1.01, p=0.42)).
Figure 1.
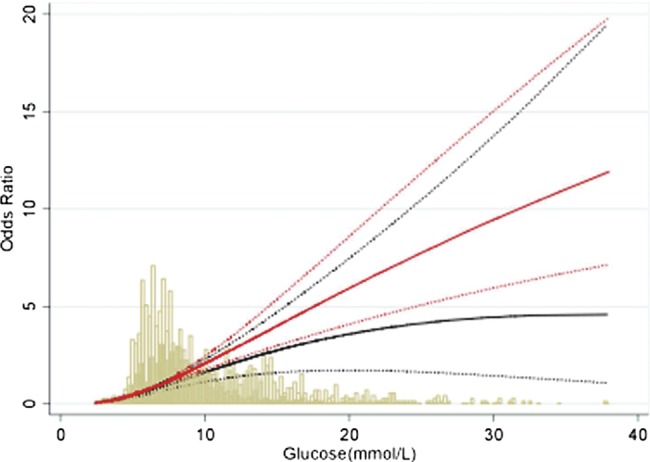
Unadjusted odds of 30-day mortality according to admission blood glucose concentration in people with and without diabetes. The bars represent the number of people at various glucose levels. Solid lines indicate OR while dotted lines indicate 95% CI. Solid bars and black lines indicate patients with diabetes. Clear bars and red lines indicate patients without diabetes.
Diabetes and glucose after AMI: influence on mortality in STEMI and NSTEMI
After adjustment for covariates, diabetes showed no statistically significant association with mortality at any time period, either for STEMI or NSTEMI (table 4). The strength of association between blood glucose and mortality was very similar in the first 30 days after STEMI or NSTEMI. The strength of this relationship declined with time only after NSTEMI.
Table 4.
Multivariate association of clinical variables with 30-day, 1-year and total mortality in the subgroups of patients with STEMI and NSTEMI
| N=4111 | Mortality, N (%) |
||||||
|---|---|---|---|---|---|---|---|
| STEMI | NSTEMI | 30 days |
1 year |
All |
|||
| 2397 | 1714 | STEMI | NSTEMI | STEMI | NSTEMI | STEMI | NSTEMI |
| Admission demographics | |||||||
| Age (year) | 1.055 (1.033 to 1.077) | 1.073 (1.031 to 1.116) | 1.061 (1.044 to 1.078) | 1.056 (1.035 to 1.079) | 1.077 (1.062 to 1.091) | 1.061 (1.046 to 1.077) | |
| SBP (mm Hg) | 0.988 (0.982 to 0.994) | 0.983 (0.970 to 0.995) | 0.992 (0.987 to 0.997) | 0.988 (0.982 to 0.995) | 0.993 (0.989 to 0.997) | 0.994 (0.990 to 0.998) | |
| Heart rate (beat/min) | 1.008 (1.001 to 1.015) | 1.008 (0.997 to 1.02) | 1.008 (1.002 to 1.013) | 1.007 (1.001 to 1.013) | 1.008 (1.004 to 1.012) | 1.007 (1.002 to 1.011) | |
| eGFR (ml/min) | 0.986 (0.975 to 0.997) | 0.987 (0.969 to 1.005) | 0.982 (0.974 to 0.991) | 0.978 (0.968 to 0.989) | 0.986 (0.979 to 0.993) | 0.987 (0.979 to 0.995) | |
| Admission plasma glucose (mmol/l) | 1.070 (1.034 to 1.107) | 1.074 (1.005 to 1.148) | 1.071 (1.042 to 1.10) | 1.021 (0.979 to 1.066) | 1.076 (1.051 to 1.10) | 1.014 (0.983 to 1.047) | |
| Prior diabetes | 1.035 (0.652 to 1.641) | 0.629 (0.264 to 1.502) | 1.083 (0.772 to 1.518) | 0.878 (0.566 to 1.36) | 1.189 (0.907 to 1.559) | 1.055 (0.773 to 1.44) | |
| Admission treatment (yes vs no) | |||||||
| Loop diuretic | 1.330 (0.890 to 1.989) | 1.66 (0.759 to 3.629) | 1.706 (1.248 (2.333) | 1.988 (1.283 to 3.081) | 1.365 (1.068 to 1.745) | 2.03 (1.496 to 2.756) | |
| Discharge medication (yes vs no) | |||||||
| Aspirin | 0.301 (0.135 to 0.672) | 0.308 (0.088 to 1.076) | 0.499 (0.322 to 0.773) | 0.869 (0.523 to 1.433) | 0.697 (0.501 to 0.970) | 1.052 (0.711 to 1.557) | |
| β-Blocker | 0.208 (0.095 to 0.455) | 0.337 (0.094 to 1.207) | 0.469 (0.320 to 0.687) | 0.77 (0.485 to 1.222) | 0.520 (0.393 to 0.698) | 0.939 (0.674 to 1.308) | |
| Statin | 1.046 (0.375 to 2.918) | 0.255 (0.066 to 0.992) | 0.551 (0.334 to 0.908) | 0.745 (0.449 to 1.237) | 0.615 (0.429 to 0.880) | 0.65 (0.444 to 0.951) | |
| ACE inhibitor or ARB | 0.392 (0.153 to 1.006) | 0.451 (0.121 to 1.673) | 0.903 (0.545 to 1.496) | 0.541 (0.348 to 0.841) | 1.041 (0.712 to 1.523) | 0.857 (0.616 to 1.194) | |
Data are HR (95% CI).
ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate calculated using the MDRD formula; MDRD, modification of diet in renal disease; NSTEMI, non-ST elevation myocardial infarction; SBP, systolic blood pressure; STEMI, ST elevation myocardial infarction.
Discussion
It is well known that both prior diabetes diagnosis and admission blood glucose concentration are associated with adverse outcome after AMI. In this report, we compared the relative association of these two measures of dysglycaemia with survival after STEMI as well as NSTEMI. Irrespective of the type of AMI, the univariate association with mortality risk for antecedent diabetes (40% excess at 30 days, 55–65% thereafter) was no longer apparent after adjustment for relevant covariates including admission glucose concentration. In contrast, the excess risk associated with increasing glucose was not reduced after adjustment, was similar in those with and without known diabetes, and remained relevant in patients discharged alive from the index event.
In our previous report of over 4000 patients with STEMI, admitted in 1993–2004,9 the 50% increase in 30-day and 1-year mortality risk associated with known diabetes was attenuated by half on covariate adjustment and removed completely when admission blood glucose concentration was included in the analysis. The current report confirms these observations and extends them to a contemporary period, and to patients with NSTEMI as well as STEMI, in whom the strength of association between admission blood glucose concentration and 30-day mortality risk was similar, and concentration dependent. Importantly, the excess risk, around 7% for each 1 mmol/l increase in admission glucose concentration, was maintained up to and beyond 1 year from the index infarction. Further, this phenomenon was attenuated with time only for patients with NSTEMI, and was evident even in those patients who survived to discharge from hospital, two potentially important clinical observations. These findings are in contrast to one previous report which reported the association between admission glucose and mortality to be confined to in-hospital deaths following either STEMI or NSTEMI.8 They are however in keeping with the vast majority of reports in this area.1–7 9 11
In contrast to most previous reports,1–9 11 we observed no independent association between diabetes and mortality risk after AMI. However, to our knowledge and unlike the present report, none of these studies adjusted for admission blood glucose, and each reported individual relationships between mortality after AMI and either diabetes diagnosis1 2 4 8 or blood glucose concentration.3–8 11–13 21 The current analysis and our previous study9 are the only reports to compare the relative association with outcome of both diabetes and blood glucose concentration. Both studies demonstrate a much stronger relationship between survival and blood glucose, and the loss of association between mortality and diabetes when blood glucose is considered. Owing to incomplete data and lack of power, we could not assess whether outcomes varied by diabetes therapies. However, previous studies have reported an independent association of admission blood glucose with mortality regardless of diabetic therapy used.2 5 7
These observations are of potential clinical significance. While admission blood glucose concentration after AMI is on average higher in patients with, compared to those without, known diabetes,4 8 9 there is considerable overlap, as seen in the current report (figure 1). While many patients presenting with AMI will have previously undiagnosed diabetes,22 blood glucose at the time of admission with AMI is not a reliable indicator of the subsequent diagnosis of diabetes.23 24 In routine practice, the management of hyperglycaemia after AMI is influenced by the presence of prior diabetes diagnosis.5 In both European14 and North American6 settings, the majority (>65%) of patients presenting with hyperglycaemia in the context of AMI, and not previously known to have diabetes, do not receive active management of blood glucose. In the presence of a true, direct toxic effect upon prognosis of elevated blood glucose, failure to correct hyperglycaemia may represent suboptimal clinical care, and patients without known diabetes may be particularly disadvantaged. In particular, our demonstration that the relationship between glucose concentration and subsequent outcome is evident in NSTEMI as well as STEMI is of clear clinical relevance in terms of the overall management of patients presenting with AMI.
The strength of association between diabetes and mortality risk after AMI has been reported to increase with time from the event.25 While we observed such a trend on univariate analysis, this was attenuated in multivariate analysis, an observation which may relate to our inclusion of blood glucose as a covariate. A previous meta-analysis suggested a stronger association between admission blood glucose and adverse outcome.4 While we could not demonstrate formal statistical evidence of such a phenomenon, our data show convincingly that the relationship between glucose and outcome is at least as powerful in patients without known diabetes. Blood glucose soon after admission represents an easily identified, clinically relevant marker of risk after AMI, which should be assessed routinely irrespective of diabetes status.
An important observation from this study is the persisting association between admission blood glucose concentration and mortality risk in patients surviving to discharge, in both NSTEMI and STEMI. While in keeping with the possibility that blood glucose concentration at admission reflects the degree of individual physiological stress, or is a marker of the extent of infarction, our findings are as much in keeping with a direct, adverse influence on prognosis of acute hyperglycaemia. The mechanisms by which elevated glucose may be directly cardiotoxic have been summarised elsewhere10 and include attenuation of ischaemic preconditioning, QT prolongation, increased thrombophilia and endothelial dysfunction. Furthermore, clinical studies overwhelmingly support a possible causal link between hyperglycaemia and adverse prognosis after AMI. Hyperglycaemia persisting at 24 h after admission is associated with adverse outcome.12 13 17
While observational studies show consistently the adverse association between hyperglycaemia and outcomes post-AMI, results of the RCTs of active management of blood glucose have been inconsistent.16 17 However, in such trials, effective reduction in blood glucose with an intervention after AMI was associated with improved prognosis.16 The guidelines from professional societies in this area differ in their recommendations.26 27 In the North American guidelines, intensive glucose control is recommended in patients with AMI and significant hyperglycaemia (blood glucose levels >10.0 mmol/l) admitted in an intensive care unit.27 In contrast, the National Institute for Health and Clinical Excellence guidance recommends against routine use of intensive insulin therapy to manage hyperglycaemia (blood glucose levels >11.0 mmol/l) in patients with acute coronary syndrome.26 The latter guidelines highlighted a need for randomised controlled trials addressing specific gaps in knowledge this area.
Our report is subject to the limitations inherent in all observational cohort studies. Our results are from a single-centre study. In the early years of the MINAP project, data on only STEMI were collected. Furthermore, data collected for MINAP was gathered mainly from a setting of coronary care unit. Selection bias could be the reason behind the overall low numbers of AMI cases (4111) recruited in our study over a 6-year period in a catchment population of one million. However, baseline and clinical outcome parameters in our study are similar to previous studies. Selection bias could also explain relatively high proportion of patients with STEMI (58.4%) compared to NSTEMI in our cohort. We therefore conducted subgroup analysis for people with STEMI and NSTEMI and compared their outcomes. Blood glucose concentration used in this analysis was that first recorded for the index admission, and is likely to have varied in timing relative to symptom onset. Our database lacks information on left ventricular (LV) ejection fraction, evidence of heart failure and a number of other potentially relevant variables. Information on body mass index, an indicator of underlying metabolic syndrome and associated dysglycaemia, was not available. Further, we have no information regarding the number of patients who were given a diagnosis of diabetes during, or subsequent to, the index admission. However, if elevated glucose contributes directly to prognosis, active management is likely to confer greater benefit when delivered as early as possible, irrespective of subsequent diabetes status. Thus, we suggest the first-recorded blood glucose concentration to be highly relevant to guiding appropriate management in individual patients, irrespective of residual LV function. While we have no information on interventions or changes to therapy after discharge, it is unlikely that these impacted on outcome in a major way, as the strongest association between mortality and glucose was in the first 30 days. Findings of our study based on real-life practice are applicable to other populations treated in similar setting.
In summary, admission blood glucose concentration is a powerful, routinely available marker of mortality risk after AMI. After adjustment for admission blood glucose, known diabetes is not associated with adverse outcome. The association between blood glucose concentration and mortality risk is of similar magnitude in patients with and without known diabetes, is evident for NSTEMI as well as STEMI, and persists beyond 1 year from the index event, including in patients surviving to discharge. Future studies are merited of the impact of active management of blood glucose in patients with all presentations of acute coronary artery disease, irrespective of diabetes diagnosis.
Supplementary Material
Footnotes
Contributors: NG, IS and KK conceived the idea of the study and were responsible for the design of the study. NG and RM were responsible for undertaking for the data analysis and produced the tables and graphs. IS, KK and MJD provided input into the data analysis. The initial draft of the manuscript was prepared by NG and IS and then circulated repeatedly among all authors for critical revision. IS was responsible for the acquisition of the data and IS, NG, RM, KK and MJD contributed to the interpretation of the results.
Funding: The work in this paper is part of the research portfolio supported by the Leicester NIHR Biomedical Research Unit in Cardiovascular Disease. NG has received support by the National Institute for Health Research, Collaboration for Leadership in Applied Health Research and Care—Leicestershire, Northamptonshire and Rutland (NIHR CLAHRC for LNR) project for a PhD.
Competing interests: None.
Ethics approval: The study was approved by the local research ethics committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There are no additional data available.
References
- 1.Malmberg K, Yusuf S, Gerstein HC, et al. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: results of the OASIS Registry. Circulation 2000;102:1014–19 [DOI] [PubMed] [Google Scholar]
- 2.McGuire DK, Emanuelsson H, Granger CB, et al. Influence of diabetes mellitus on clinical outcomes across the spectrum of acute coronary syndromes. Findings from the GUSTO IIb study. Eur Heart J 2000;21:1750–8 [DOI] [PubMed] [Google Scholar]
- 3.Svensson A-M, McGuire DK, Abrahamsson P, et al. Association between hyper- and hypoglycaemia and 2-year all-cause mortality risk in diabetic patients with acute coronary events. Eur Heart J 2005;26:1255–61 [DOI] [PubMed] [Google Scholar]
- 4.Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet 2000;355:773–8 [DOI] [PubMed] [Google Scholar]
- 5.Wahab NN, Cowden EA, Pearce NJ, et al. Is blood glucose an independent predictor of mortality in acute myocardial infarction in the thrombolytic era? J Am Coll Cardiol 2002;40:1748–54 [DOI] [PubMed] [Google Scholar]
- 6.Kosiborod M, Rathore SS, Inzucchi SE, et al. Admission glucose and mortality in elderly patients hospitalised with acute myocardial infarction. Circulation 2005;111:3078–86 [DOI] [PubMed] [Google Scholar]
- 7.Cao JJ, Hudson M, Jankowski M, et al. Relation of chronic and acute glycaemic control on mortality in acute myocardial infarction with diabetes mellitus. Am J Cardiol 2005;96:183–6 [DOI] [PubMed] [Google Scholar]
- 8.Sinnaeve PR, Steg G, Fox KAA, et al. Association of fasting glucose with increased short-term and 6-month mortality in ST-elevation and non ST-elevation acute coronary syndromes. Arch Int Med 2009;169:402–9 [DOI] [PubMed] [Google Scholar]
- 9.Squire IB, Nelson CP, Ng LL, et al. Prognostic value of admission blood glucose concentration and diabetes diagnosis on survival after acute myocardial infarction; results from 4702 index cases in routine practice. Clin Sci (London) 2010;118:527–35 [DOI] [PubMed] [Google Scholar]
- 10.Ceriello A. Acute hyperglycaemia: a new risk factor during myocardial infarction. Eur Heart J 2001;26:328–31 [DOI] [PubMed] [Google Scholar]
- 11.De Caterina R, Madonna R, Sourij H, et al. Glycaemia control in acute coronary syndromes: prognostic value and therapeutic options. Eur Heart J 2010;31:1557–64 [DOI] [PubMed] [Google Scholar]
- 12.Ghoyal A, Mahaffey KW, Garg J, et al. Prognostic significance of the change in glucose level in the first 24h after acute myocardial infarction: results from the CARDINAL study. Eur Heart J 2006;27:1289–97 [DOI] [PubMed] [Google Scholar]
- 13.Norhammar AM, Ryden L, Malmberg K. Admission plasma glucose. Independent risk factor for long-term prognosis after myocardial infarction even in non-diabetic patients. Diabetes Care 1999;22:1827–31 [DOI] [PubMed] [Google Scholar]
- 14.Weston C, Walker L, Birkhead J. Early impact of insulin treatment on mortality for hyperglycaemic patients without known diabetes who present with an acute coronary syndrome. Heart 2007;93:1542–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schnell O, Schafer O, Kleybrink S, et al. Intensification of therapeutic approaches reduces mortality in diabetic patients with acute myocardial infarction: the Munich registry. Diabetes Care 2004;27:455–60 [DOI] [PubMed] [Google Scholar]
- 16.Malmberg K, Ryden L, Efendic S, et al. Randomised trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol 1995;26:57–65 [DOI] [PubMed] [Google Scholar]
- 17.Malmberg K, Ryden L, Wedel H, et al. , DIGAMI 2 Investigators Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J 2005;26:650–61 [DOI] [PubMed] [Google Scholar]
- 18.Herrett E, Smeeth L, Walker L, et al. MINAP Academic group The Myocardial Ischaemia National Audit Project (MINAP). Heart 2010;96:1264–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blackledge HM, Newton J, Squire IB. Prognosis for South Asian and white patients newly admitted to hospital with heart failure in the United Kingdom: historical cohort study. BMJ 2003;327:526–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thygesen K, Alpert JS, White HD. The Joint ESC/ACCF/AHA/WHF Task Force for the redefinition of myocardial infarction . Eur Heart J 2007;28:2525–38 [DOI] [PubMed] [Google Scholar]
- 21.Kosiborod M, Inzucchi SE, Krumholz HM, et al. Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes-based measure of risk. Circulation 2008;117:1018. [DOI] [PubMed] [Google Scholar]
- 22.Norhammar A, Tenerz A, Nilsson G, et al. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet 2002;359:2140–4 [DOI] [PubMed] [Google Scholar]
- 23.Tenerz A, Lonnberg I, Berne C, et al. Myocardial infarction and prevalence of diabetes mellitus, Is increased casual blood glucose at admission a reliable criterion for the diagnosis of diabetes? Eur Heart J 2001;22:1102–10 [DOI] [PubMed] [Google Scholar]
- 24.De Mulder M, Oemrawsingh RH, Stam F, et al. Comparison of diagnostic criteria to detect undiagnosed diabetes in hyperglycaemiac patients with acute coronary syndrome. Heart 2012;98:37–41. [DOI] [PubMed] [Google Scholar]
- 25.Melchior T, Kober L, Madsen CR, et al. Accelerating impact of diabetes mellitus on mortality in the years following an acute myocardial infarction. Eur Heart J 1999;20:973–8 [DOI] [PubMed] [Google Scholar]
- 26.National Institute for Health and Clinical Excellence Hyperglycaemia in acute coronary syndromes: management of hyperglycaemia in people with acute coronary syndromes (CG 130). London: National Institute for Health and Clinical Excellence, 2011 [PubMed] [Google Scholar]
- 27.Deedwania P, Kosibirod M, Barrett E, et al. Hyperglycaemia and acute coronary syndrome. A scientific Statement from the American Heart Association Diabetes Committee of the Council on Nutrition, Physical activity and Metabolism. Circulation 2008;117:1610–19 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


