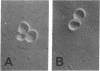
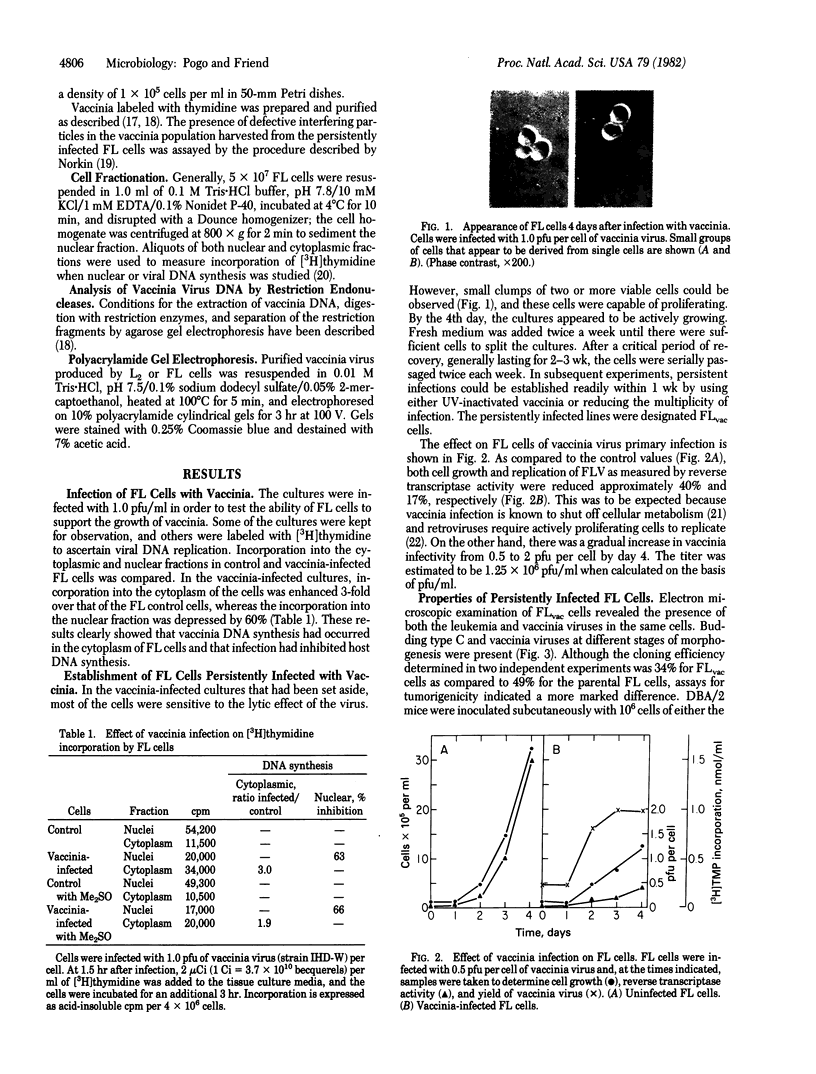
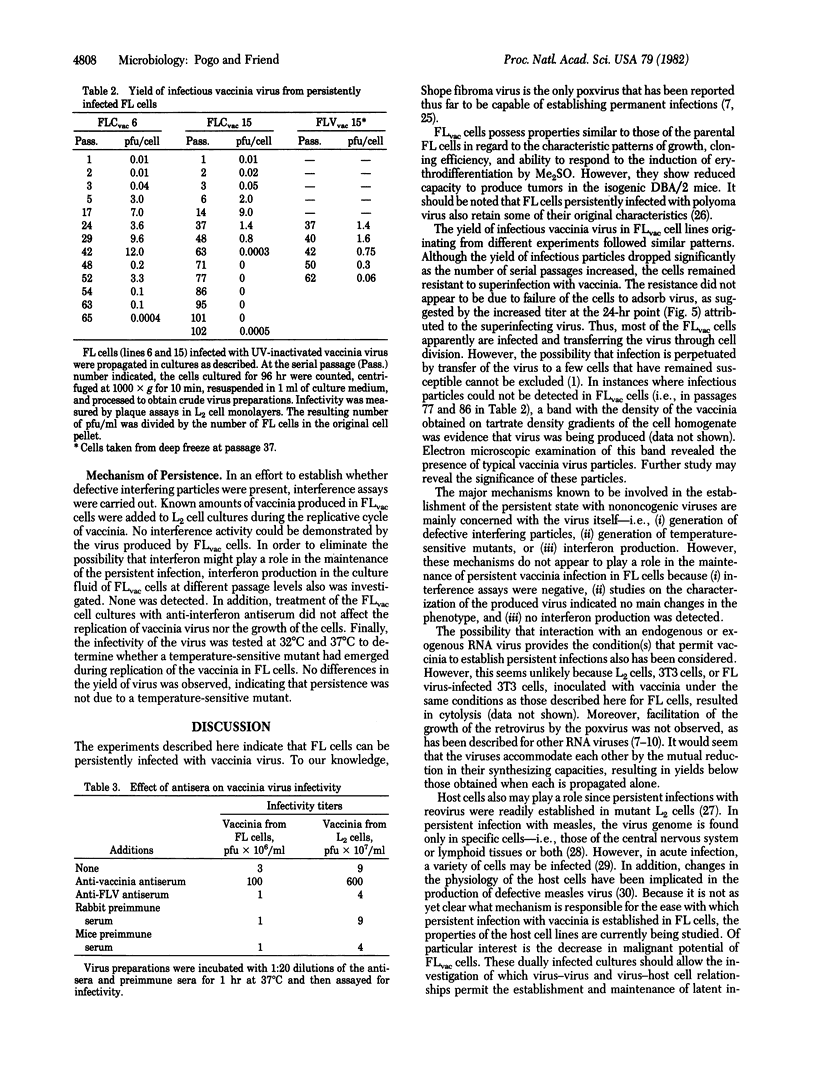
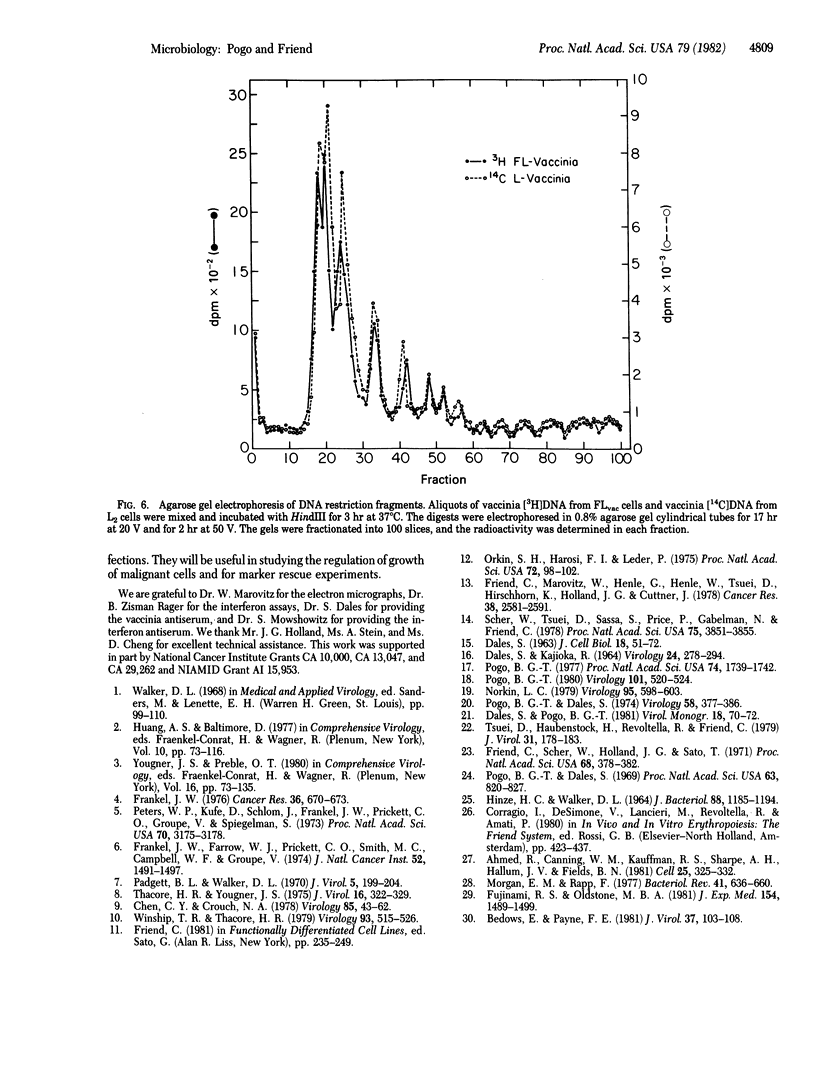
Abstract
Cultures of murine Friend erythroleukemia (FL) cells, which are chronically infected with leukemia virus, were inoculated with vaccinia virus. The yield of vaccinia virus was determined by assaying plaque-forming units in mouse L2 cells, and the yield of leukemia virus was determined by measuring reverse transcriptase (RNA-dependent DNA nucleotidyltransferase) activity released into the culture fluid. Although no facilitation of one virus by the other was detected, persistently infected cultures were established. Electron microscopic examination revealed the presence of vaccinia and leukemia viruses in the same cell. The permanent lines of cells persistently infected with vaccinia were designated FLvac. Their morphology, growth rate, cloning efficiency, and ability to respond to the induction of erythrodifferentiation by treatment with dimethyl sulfoxide were not appreciably altered as compared to the parental FL cells. However, the persistently infected cells showed a marked decrease in tumorigenicity when assayed in DBA/2 mice. The infectious virus produced by FLvac cells and by L2 cells were indistinguishable as judged by restriction endonuclease patterns of virion DNA, structural proteins, and the activities of two virion-associated DNases. The yield of infectious vaccinia virus from FLvac cells generally declined after about 60 serial passages. Although some cell lines no longer yield infectious virus, they are resistant to challenge with vaccinia at concentrations that are cytolytic for L2 cells. The mechanism responsible for the establishment of the persistent infection remains unclear because defective particles, interferon production, and temperature-sensitive mutants have not been detected.
Full text
PDF
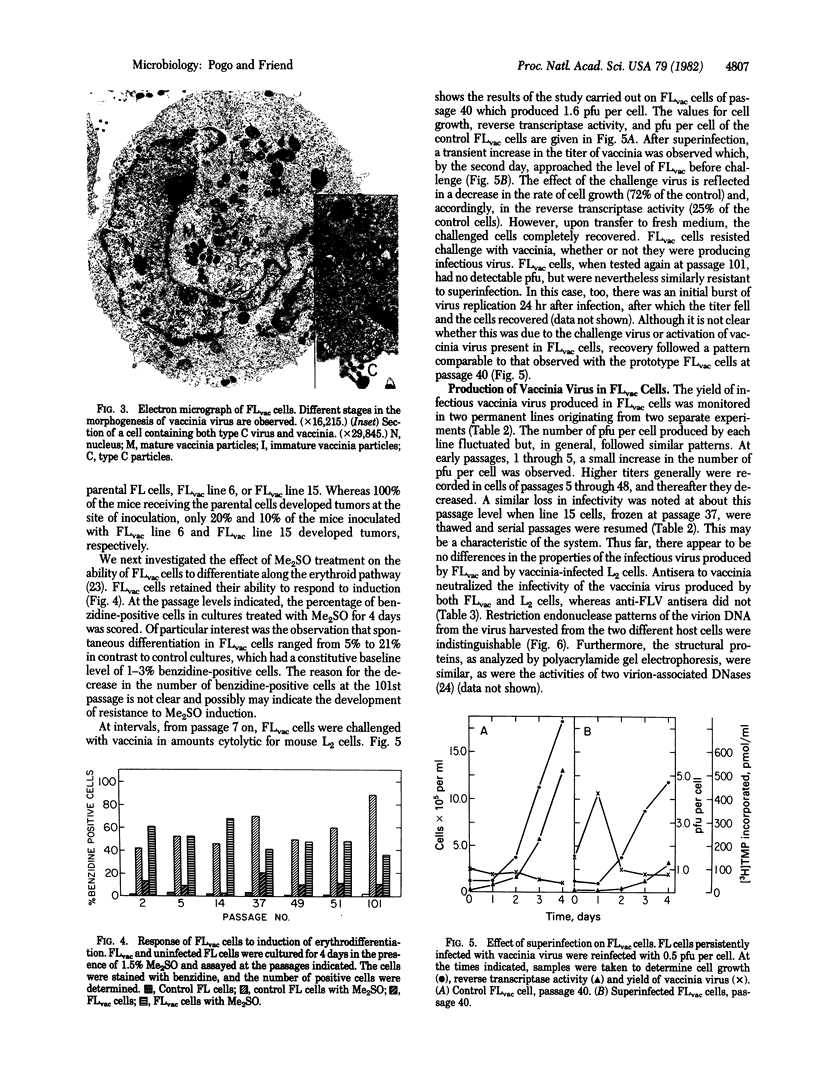
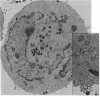
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Canning W. M., Kauffman R. S., Sharpe A. H., Hallum J. V., Fields B. N. Role of the host cell in persistent viral infection: coevolution of L cells and reovoirus during persistent infection. Cell. 1981 Aug;25(2):325–332. doi: 10.1016/0092-8674(81)90050-7. [DOI] [PubMed] [Google Scholar]
- Bedows E., Payne F. E. Host cell factors involved in the production of slowly sedimenting nucleocapsids in measles virus-infected cells. J Virol. 1981 Jan;37(1):103–108. doi: 10.1128/jvi.37.1.103-108.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Y., Crouch N. A. Shope fibroma virus-induced facilitation of vesicular stomatitis virus adsorption and replication in nonpermissive cells. Virology. 1978 Mar;85(1):43–62. doi: 10.1016/0042-6822(78)90410-5. [DOI] [PubMed] [Google Scholar]
- DALES S., KAJIOKA R. THE CYCLE OF MULTIPLICATION OF VACCINIA VIRUS IN EARLE'S STRAIN L CELLS. I. UPTAKE AND PENETRATION. Virology. 1964 Nov;24:278–294. doi: 10.1016/0042-6822(64)90167-9. [DOI] [PubMed] [Google Scholar]
- DALES S. The uptake and development of vaccinia virus in strain L cells followed with labeled viral deoxyribonucleic acid. J Cell Biol. 1963 Jul;18:51–72. doi: 10.1083/jcb.18.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel J. W. Experimental models for DNA-RNA viral interactions: a brief review. Cancer Res. 1976 Feb;36(2 Pt 2):670–673. [PubMed] [Google Scholar]
- Frankel J. W., Farrow W. M., Prickett C. O., Smith M. E., Campbell W. F., Groupè V. Responses of isolator-derived and conventional chickens to Marek's disease herpesvirus and avian leukosis virus. J Natl Cancer Inst. 1974 May;52(5):1491–1497. doi: 10.1093/jnci/52.5.1491. [DOI] [PubMed] [Google Scholar]
- Friend C., Marovitz W., Henie G., Henie W., Tsuei D., Hirschhorn K., Holland J. G., Cuttner J. Observations on cell lines derived from a patient with Hodgkin's disease. Cancer Res. 1978 Aug;38(8):2581–2591. [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B. Failure to cleave measles virus fusion protein in lymphoid cells. J Exp Med. 1981 Nov 1;154(5):1489–1499. doi: 10.1084/jem.154.5.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HINZE H. C., WALKER D. L. RESPONSE OF CULTURED RABBIT CELLS TO INFECTION WITH THE SHOPE FIBROMA VIRUS. I. PROLIFERATION AND MORPHOLOGICAL ALTERATION OF THE INFECTED CELLS. J Bacteriol. 1964 Oct;88:1185–1194. doi: 10.1128/jb.88.4.1185-1194.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E. M., Rapp F. Measles virus and its associated diseases. Bacteriol Rev. 1977 Sep;41(3):636–666. doi: 10.1128/br.41.3.636-666.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin L. C. The emergence of simian virus 40 variants in a persistent infection of rhesus monkey kidney cells and their interaction with standard simian virus 40. Virology. 1979 Jun;95(2):598–603. doi: 10.1016/0042-6822(79)90515-4. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Harosi F. I., Leder P. Differentiation in erythroleukemic cells and their somatic hybrids. Proc Natl Acad Sci U S A. 1975 Jan;72(1):98–102. doi: 10.1073/pnas.72.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L. Effect of persistent fibroma virus infection on susceptibility of cells to other viruses. J Virol. 1970 Feb;5(2):199–204. doi: 10.1128/jvi.5.2.199-204.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters W. P., Kufe D., Schlom J., Frankel J. W., Prickett C. O., Groupé V., Spiegelman S. Biological and biochemical evidence for an interaction between Marek's disease herpesvirus and avian leukosis virus in vivo. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3175–3178. doi: 10.1073/pnas.70.11.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G. Changes in parental vaccinia virus DNA after viral penetration into cells. Virology. 1980 Mar;101(2):520–524. doi: 10.1016/0042-6822(80)90466-3. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Biogenesis of poxviruses: further evidence for inhibition of host and virus DNA synthesis by a component of the invading inoculum particle. Virology. 1974 Apr;58(2):377–386. doi: 10.1016/0042-6822(74)90073-7. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Two deoxyribonuclease activities within purified vaccinia virus. Proc Natl Acad Sci U S A. 1969 Jul;63(3):820–827. doi: 10.1073/pnas.63.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G. Elimination of naturally occurring crosslinks in vaccinia virus DNA after viral penetration into cells. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1739–1742. doi: 10.1073/pnas.74.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher W., Tsuei D., Sassa S., Price P., Gabelman N., Friend C. Inhibition of dimethyl sulfoxide-stimulated Friend cell erythrodifferentiation by hydrocortisone and other steroids. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3851–3855. doi: 10.1073/pnas.75.8.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacore H. R., Youngner J. S. Abortive infection of a rabbit cornea cell line by vesicular stomatitis virus: conversion to productive infection by superinfection with vaccinia virus. J Virol. 1975 Aug;16(2):322–329. doi: 10.1128/jvi.16.2.322-329.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuei D., Haubenstock H., Revoltella R., Friend C. Virus production and hemoglobin synthesis in variant lines of dimethyl sulfoxide-treated Friend erythroleukemia cells. J Virol. 1979 Jul;31(1):178–183. doi: 10.1128/jvi.31.1.178-183.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winship T. R., Thacore H. R. Inhibition of vesicular stomatitis virus-defective interfering particle synthesis by Shope fibroma virus. Virology. 1979 Mar;93(2):515–526. doi: 10.1016/0042-6822(79)90254-x. [DOI] [PubMed] [Google Scholar]