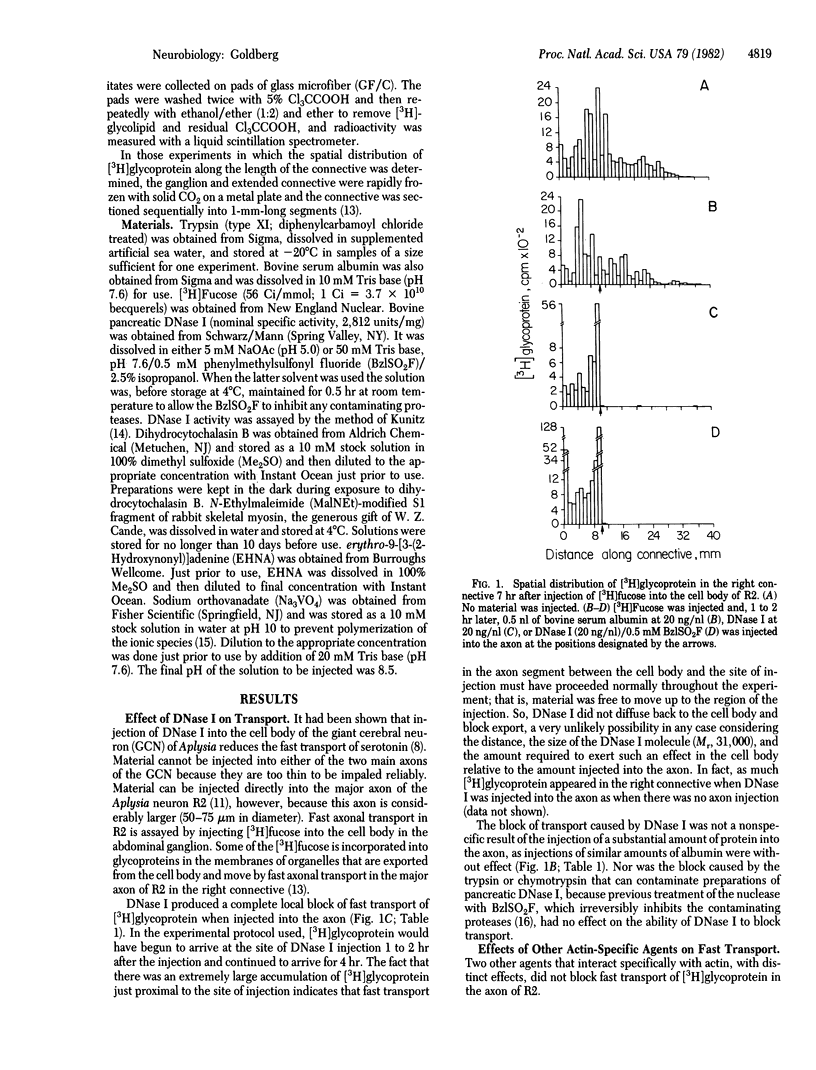
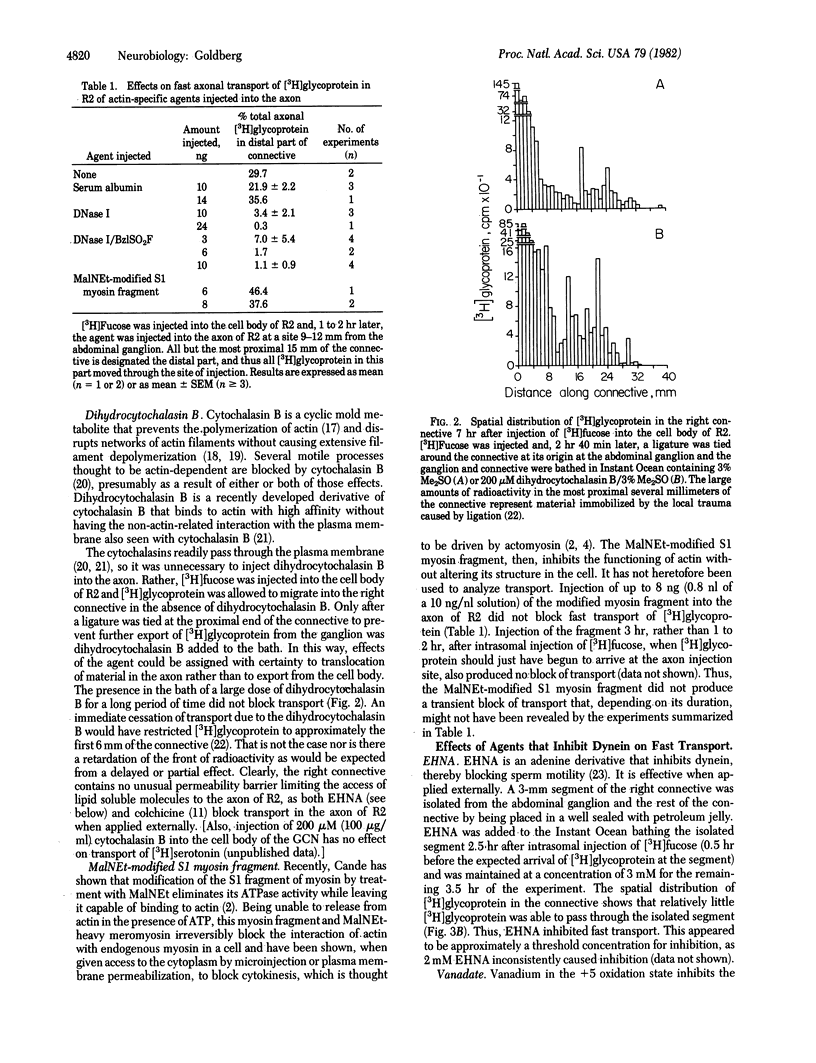
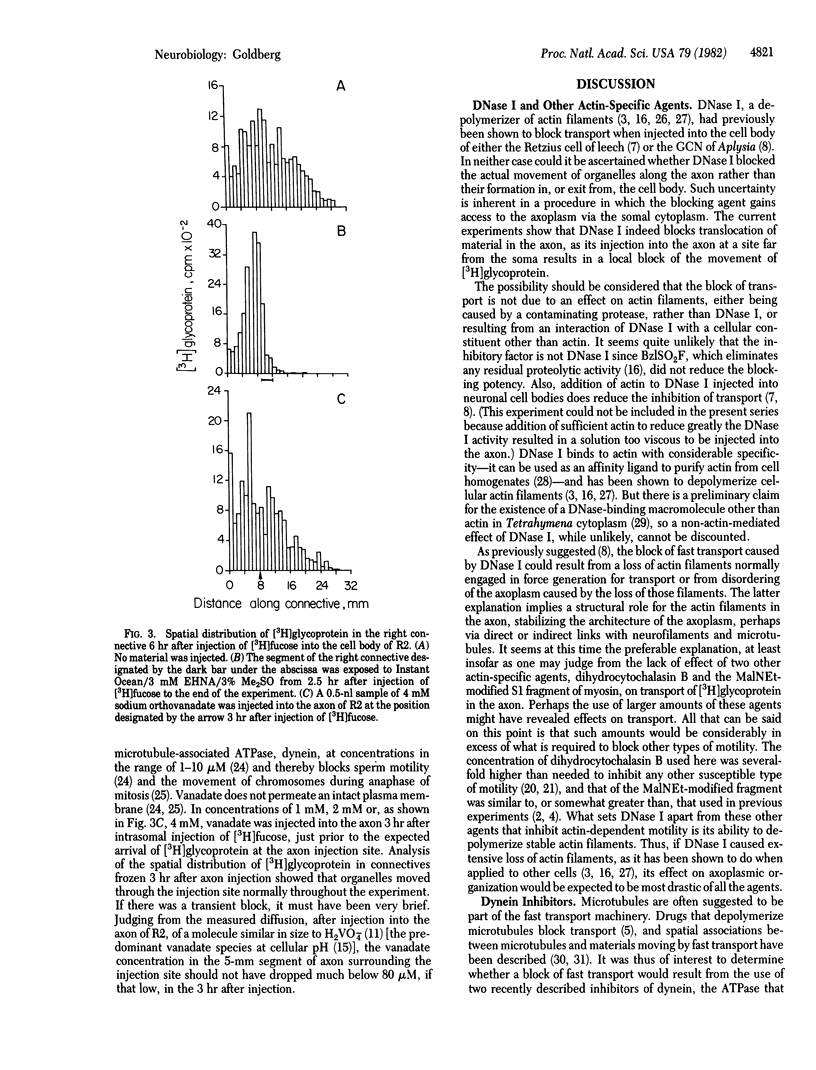
Abstract
Microinjection into an axon of an identified invertebrate neuron is shown to be a useful technique for analyzing the mechanisms of fast axonal transport. It permits direct assessment of the effect of agents that cannot permeate the plasma membrane on the translocation of material in the axon. The actin filament depolymerizer DNase I, when injected into the axon of the Aplysia neuron R2, caused a local block of fast transport of [3H]glycoprotein. Two agents that should interfere with the functioning of actin filaments without causing extensive depolymerization, tne N-ethylmaleimide-modified nuclease S1 fragment of myosin (injected) and dihydrocytochalasin B (applied externally). had no effect. Together these results suggest that actin plays a structural role in the axonal cytoskeleton rather than a role in transport force generation, the effect of DNase I being mediated by structural disordering of the axoplasm. Experiments were also done with inhibitors of dynein, the microtubule-associated ATPase. erythro-9-[3-(2-Hydroxynonyl)]adenine blocked transport but vanadate was ineffective.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambron R. T., Goldman J. E., Schwartz J. H. Axonal transport of newly synthesized glycoproteins in a single identified neuron of Aplysia californica. J Cell Biol. 1974 Jun;61(3):665–675. doi: 10.1083/jcb.61.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambron R. T., Goldman J. E., Thompson E. B., Schwartz J. H. Synthesis of glycoproteins in a single identified neuron of Aplysia californica. J Cell Biol. 1974 Jun;61(3):649–664. doi: 10.1083/jcb.61.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckerle M. C., Porter K. R. Inhibitors of dynein activity block intracellular transport in erythrophores. Nature. 1982 Feb 25;295(5851):701–703. doi: 10.1038/295701a0. [DOI] [PubMed] [Google Scholar]
- Bouchard P., Penningroth S. M., Cheung A., Gagnon C., Bardin C. W. erythro-9-[3-(2-Hydroxynonyl)]adenine is an inhibitor of sperm motility that blocks dynein ATPase and protein carboxylmethylase activities. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1033–1036. doi: 10.1073/pnas.78.2.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. S., Spudich J. A. Cytochalasin inhibits the rate of elongation of actin filament fragments. J Cell Biol. 1979 Dec;83(3):657–662. doi: 10.1083/jcb.83.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cande W. Z. A permeabilized cell model for studying cytokinesis using mammalian tissue culture cells. J Cell Biol. 1980 Nov;87(2 Pt 1):326–335. doi: 10.1083/jcb.87.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cande W. Z., Wolniak S. M. Chromosome movement in lysed mitotic cells is inhibited by vanadate. J Cell Biol. 1978 Nov;79(2 Pt 1):573–580. doi: 10.1083/jcb.79.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstadt M., Goldman J. E., Kandel E. R., Koike H., Koester J., Schwartz J. H. Intrasomatic injection of radioactive precursors for studying transmitter synthesis in identified neurons of Aplysia californica. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3371–3375. doi: 10.1073/pnas.70.12.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons B. H., Gibbons I. R. The effect of partial extraction of dynein arms on the movement of reactivated sea-urchin sperm. J Cell Sci. 1973 Sep;13(2):337–357. doi: 10.1242/jcs.13.2.337. [DOI] [PubMed] [Google Scholar]
- Gibbons I. R., Cosson M. P., Evans J. A., Gibbons B. H., Houck B., Martinson K. H., Sale W. S., Tang W. J. Potent inhibition of dynein adenosinetriphosphatase and of the motility of cilia and sperm flagella by vanadate. Proc Natl Acad Sci U S A. 1978 May;75(5):2220–2224. doi: 10.1073/pnas.75.5.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D. J., Ambron R. T. Two rates of fast axonal transport of [3H]glycoprotein in an identified invertebrate neuron. Brain Res. 1981 Dec 21;229(2):445–455. doi: 10.1016/0006-8993(81)91006-4. [DOI] [PubMed] [Google Scholar]
- Goldberg D. J., Harris D. A., Lubit B. W., Schwartz J. H. Analysis of the mechanism of fast axonal transport by intracellular injection of potentially inhibitory macromolecules: evidence for a possible role of actin filaments. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7448–7452. doi: 10.1073/pnas.77.12.7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafstein B., Forman D. S. Intracellular transport in neurons. Physiol Rev. 1980 Oct;60(4):1167–1283. doi: 10.1152/physrev.1980.60.4.1167. [DOI] [PubMed] [Google Scholar]
- Hartwig J. H., Stossel T. P. Cytochalasin B and the structure of actin gels. J Mol Biol. 1979 Nov 5;134(3):539–553. doi: 10.1016/0022-2836(79)90366-8. [DOI] [PubMed] [Google Scholar]
- Hitchcock S. E., Carisson L., Lindberg U. Depolymerization of F-actin by deoxyribonuclease I. Cell. 1976 Apr;7(4):531–542. doi: 10.1016/0092-8674(76)90203-8. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Schubert P., Kreutzberg G. W. Experimental approach to test the role of actin in axonal transport. Brain Res. 1980 Aug 4;194(2):588–593. doi: 10.1016/0006-8993(80)91247-0. [DOI] [PubMed] [Google Scholar]
- KUNITZ M. Crystalline desoxyribonuclease; isolation and general properties; spectrophotometric method for the measurement of desoxyribonuclease activity. J Gen Physiol. 1950 Mar;33(4):349–362. doi: 10.1085/jgp.33.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughran L. J., Larsen J. H., Jr, Schroeder P. C. Microfilaments interacting with heavy meromyosin and deoxyribonuclease I in cells of the ovarian follicle of a lizard. Cell Tissue Res. 1981;218(3):537–545. doi: 10.1007/BF00210113. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Lindberg U. Actin is the naturally occurring inhibitor of deoxyribonuclease I. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4742–4746. doi: 10.1073/pnas.71.12.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Lin D. C., Flanagan M. D. Specificity of the effects of cytochalasin B on transport and motile processes. Proc Natl Acad Sci U S A. 1978 Jan;75(1):329–333. doi: 10.1073/pnas.75.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeusen R. L., Bennett J., Cande W. Z. Effect of microinjected N-ethylmaleimide-modified heavy meromyosin on cell division in amphibian eggs. J Cell Biol. 1980 Sep;86(3):858–865. doi: 10.1083/jcb.86.3.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papasozomenos S. C., Autilio-Gambetti L., Gambetti P. Reorganization of axoplasmic organelles following beta, beta'-iminodipropionitrile administration. J Cell Biol. 1981 Dec;91(3 Pt 1):866–871. doi: 10.1083/jcb.91.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penningroth S. M., Cheung A., Bouchard P., Gagnon C., Bardin C. W. Dynein ATPase is inhibited selectively in vitro by erythro-9-[3-2-(hydroxynonyl)]adenine. Biochem Biophys Res Commun. 1982 Jan 15;104(1):234–240. doi: 10.1016/0006-291x(82)91964-7. [DOI] [PubMed] [Google Scholar]
- Rebhun L. I. Polarized intracellular particle transport: saltatory movements and cytoplasmic streaming. Int Rev Cytol. 1972;32:93–137. doi: 10.1016/s0074-7696(08)60339-3. [DOI] [PubMed] [Google Scholar]
- Schliwa M. Action of cytochalasin D on cytoskeletal networks. J Cell Biol. 1982 Jan;92(1):79–91. doi: 10.1083/jcb.92.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P. DNase-I-dependent dissociation of erythrocyte cytoskeletons. J Cell Biol. 1979 Apr;81(1):266–270. doi: 10.1083/jcb.81.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. S., Järlfors U., Cameron B. F. Morphological evidence for the participation of microtubules in axonal transport. Ann N Y Acad Sci. 1975 Jun 30;253:472–506. doi: 10.1111/j.1749-6632.1975.tb19223.x. [DOI] [PubMed] [Google Scholar]
- Taylor D. L., Condeelis J. S., Moore P. L., Allen R. D. The contractile basis of amoeboid movement. I. The chemical control of motility in isolated cytoplasm. J Cell Biol. 1973 Nov;59(2 Pt 1):378–394. doi: 10.1083/jcb.59.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treistman S. N., Schwartz J. H. Metabolism of acetylcholine in the nervous system of Aplysia californica. IV. Studies of an identified cholinergic axon. J Gen Physiol. 1977 Jun;69(6):725–741. doi: 10.1085/jgp.69.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehland J., Weber K., Gawlitta W., Stockem W. Effects of the actin-binding protein DNAase I on cytoplasmic streaming and ultrastructure of Amoeba proteus. An attempt to explain amoeboid movement. Cell Tissue Res. 1979 Jul 17;199(3):353–372. doi: 10.1007/BF00236075. [DOI] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]



