Abstract
The delivery of high-frequency alternating currents has been shown to produce a focal and reversible conduction block in whole nerve and is a potential therapeutic option for various diseases and disorders involving pathological or undesired neurological activity. However, delivery of high-frequency alternating current to a nerve produces a finite burst of neuronal firing, called the onset response, before the nerve is blocked. Reduction or elimination of the onset response is very important to moving this type of nerve block into clinical applications since the onset response is likely to result in undesired muscle contraction and pain. This paper describes a study of the effect of nerve cuff electrode geometry (specifically, bipolar contact separation distance), and waveform amplitude on the magnitude and duration of the onset response. Electrode geometry and waveform amplitude were both found to affect these measures. The magnitude and duration of the onset response showed a monotonic relationship with bipolar separation distance and amplitude. The duration of the onset response varied by as much as 820% on average for combinations of different electrode geometries and waveform amplitudes. Bipolar electrodes with a contact separation distance of 0.5 mm resulted in the briefest onset response on average. Furthermore, the data presented in this study provide some insight into a biophysical explanation for the onset response. These data suggest that the onset response consists of two different phases: one phase which is responsive to experimental variables such as electrode geometry and waveform amplitude, and one which is not and appears to be inherent to the transition to the blocked state. This study has implications for nerve block electrode and stimulation parameter selection for clinical therapy systems and basic neurophysiology studies.
Index Terms: High-frequency alternating current, nerve conduction block, nerve cuff electrode, onset response, peripheral nerve
I. Introduction
Many diseases and disorders are characterized by pathological or undesired neural activity, including peripheral nerve pain, spasticity, and movement disorders. Arresting this activity with a focal nerve block is likely to alleviate the negative symptoms associated with many of these conditions. A quickly reversible focal nerve blockade also holds promise for enabling a new generation of neural prosthetic systems for neurogenic bladder [1], [2] and motor restoration for those with both paralysis and spasticity [3]. There has been a recent increase in the number of published studies using high-frequency alternating currents (HFAC) to achieve such a nerve block. Much of this recent work has focused on the characterization of gross nerve response to this type of current. In summary, recent work has demonstrated that HFAC produces a focal block at the site of the electrode that is quickly reversible (usually within 1 s) [4], [5] and is fast acting (usually less than a couple of seconds) [4], [6]. It has also been shown that this type of nerve block induces an intense neural volley at the onset of HFAC delivery before block occurs, which we term the onset response [2], [4]–[14]. The blocking waveform typically used in mammalian experiments is a 2–40 kHz sinusoid or square wave with an amplitude of 3–10 V (1–10 mA for current-controlled studies) [1], [2], [4], [6], [10], [11], [14], [15], with higher frequencies requiring higher amplitudes to achieve block [4], [6], [11]. A recent study in our laboratory has begun to explore the effects of electrode geometry on HFAC nerve block, demonstrating that the amplitude required to achieve block is a function of the intercontact spacing of a multipolar cuff electrode [15]. This manuscript describes a further investigation of electrode design for nerve block.
The brief but intense neural activity that occurs when HFAC is first delivered to a nerve, which we call the onset response, needs to be substantially reduced or eliminated before HFAC block can have a broad clinical application. It is desirable to prevent this activity from occurring for both motor and sensory applications, because it is likely to result in extreme pain and activation of any innervated end effector such as a muscle. This study evaluates the effect of nerve cuff electrode geometry on the onset response, namely the separation distance between two bipolar electrode contacts, as well as the effect of waveform amplitude on the onset response.
Based on data presented in the literature regarding the onset response [4], [9], [12], [16], it is apparent that the onset response is characterized by two phases that are usually distinct. The first phase, “Phase I,” is always present if neural conduction block is achieved. Phase I is characterized by a brief (tens of msec) and intense neural volley. This phase of the response has been demonstrated in whole nerve/muscle preparations [2], [4]–[6], [9]–[14], single fiber recordings [7], [8] and in single fiber neuronal simulation [5], [6], [17], [18]. This intense neural volley manifests itself as a very large muscle twitch in whole nerve/muscle preps (as shown in Fig. 1), similar to the large muscle twitch which is generated with n-let stimulation [19]. We show in this study that Phase I of the onset response is quite consistent in magnitude between trials, between animals and across the two experimental variables tested.
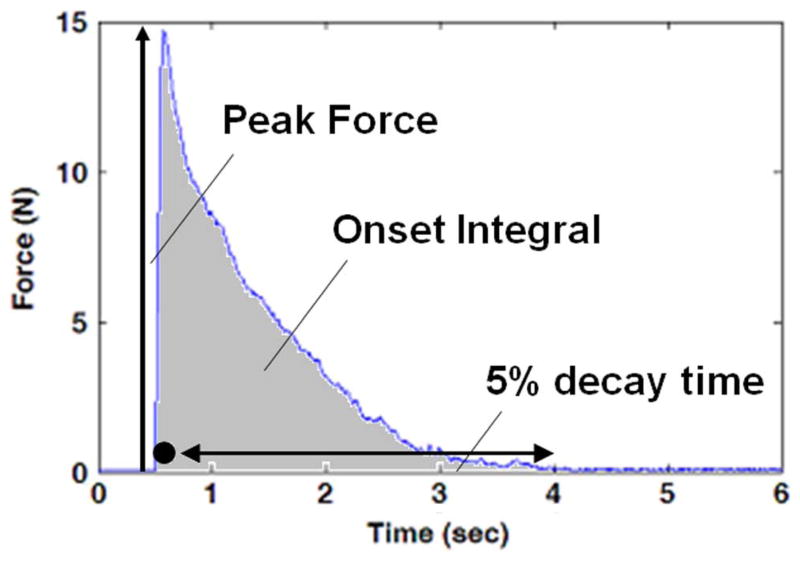
Fig. 1.
Typical gastrocnemius muscle force when HFAC is delivered to the sciatic nerve. Circle designates approximate timing of Phase I of the response. Double arrow designates approximate timing of Phase II of the onset response. Each of the three measures of onset response is schematically depicted in this trial plot.
The second phase of the onset response, “Phase II,” is usually, but not always, present [4], [5]. Phase II is characterized by repetitive firing in the nerve that occurs after the intense volley of Phase I and can last for tens of seconds. In most cases, the nerve accommodates to the HFAC and the repetitive firing diminishes gradually into quiescence. This activity has been demonstrated in both whole nerve/muscle [4]–[6], [9]–[14] and single fiber preparations [7], [8]. A typical Phase II response in a whole nerve/muscle preparation can be seen in Fig. 1. A biophysical explanation for this accommodation has not yet been proposed in the literature, and no neuronal modeling studies have been published which capture this behavior.
Several studies have shown that the magnitude of the onset response is dependent on experimental variables or conditions. Variability in the response was first observed by Rosenblueth and Reboul in 1939 who noted a shortening of the neural volley with increases in HFAC amplitude [9]. Since then, published studies have mentioned or presented data suggesting that the onset response is affected by the amplitude of the HFAC waveform [2], [4], [8], [9], [11], the frequency of the waveform [2], [4], [10]–[13], variability of the surgical preparation [4], [12], position of the blocking electrode [4], and cumulative dose of HFAC [9]. Here we investigate the effect of the geometry of a nerve cuff electrode. The effect of HFAC amplitude was also evaluated in these experiments, using multiples of the nerve block threshold rather than a fixed waveform voltage or current (for the first time in the literature). A subset of these results has also been published in abstract form [20].
Based on the previous experimental evidence, we hypothesized that the Phase II portion of the onset response could be significantly reduced or eliminated by optimizing the HFAC waveform and electrode geometry.
II. Methods
A. Surgical Preparation
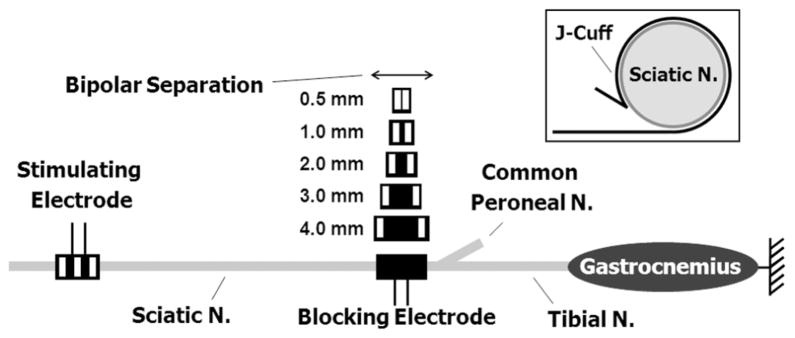
The experiments were performed in a rat sciatic nerve and gastrocnemius-soleus muscle preparation which has been used in other HFAC studies in the rat [4], [12], [14], [15]. Alpha motor fibers in the rat sciatic which innervate these muscles range from 2–13 μm in diameter [21]. Acute experiments were performed in a total of five adult Sprague–Dawley rats. All protocols involving animal use were approved by our institutional animal care and use committee. The animals were anesthetized with intraperitoneal injections of Nembutal (sodium pentobarbital). The left hind leg was shaved and an incision was made along the posterior aspect of the hind leg and thigh. The sciatic nerve was exposed from its most proximal aspect, just distal to the sacral plexus, to the popliteal fossa. The common peroneal and sural nerves were severed approximately 5–15 mm from their branch point with the sciatic nerve. The gastrocnemius-soleus muscle complex was dissected, and the calcaneal (Achilles) tendon was severed from its distal attachment at the heel. The ipsilateral tibia was stabilized to the experimental rig via a clamp, and the calcaneal tendon was tethered to a force transducer with 1–2 N of passive tension. Fig. 2 shows a diagram of the experimental setup.
Fig. 2.
Diagram of the experimental setup showing nerve cuff electrode placement. A tripolar proximal stimulating electrode was always used. HFAC blocking nerve cuff electrodes of various geometries were placed at the branch point for the common peroneal nerve for each of the block randomized trials. The inset shows a cross sectional depiction of a J-cuff electrode on the sciatic nerve.
B. Experimental Setup and Design
The effect of a bipolar nerve cuff electrode’s contact separation distance on Phase I and Phase II of the high-frequency onset response was tested in five rats. A 40-kHz HFAC blocking waveform was tested at four different amplitudes in each experiment.
For each rat, two nerve cuff electrodes were placed on the sciatic nerve as shown in Fig. 2 (the inset shows a cross sectional depiction of a cuff electrode on the sciatic nerve). Both electrodes were silicone nerve cuff electrodes with a J-shaped cross-section and rectangular platinum contacts for current delivery. This type of electrode has been used in several other studies of HFAC in whole nerve preparations [1], [4], [12], [15]. The proximal electrode was used to generate gastrocnemius-soleus muscle twitches with the delivery of 1 Hz, 20 μs supramaximal (typically 300–700 μA) cathodic pulses. These pulses were delivered using a Grass S88 (Grass Technologies, West Warwick, RI) stimulator with a current-controlled output stage. The proximal electrode was tripolar with a 2.0 mm edge-to-edge contact spacing with the outer contacts electrically tied together. Each rectangular contact had dimensions of 3 mm × 1 mm (the 1 mm dimension was along the length of the nerve). The proximal stimulating electrode was placed on the sciatic nerve within 1 cm of the sacral plexus.
A distal electrode was used to deliver the HFAC blocking current. Each blocking electrode contained two 3 mm × 1 mm contacts with a variable bipolar separation distance (the 1 mm dimension was along the length of the nerve). All blocking cuff electrodes had 1.0 mm of silicone between the outer edge of the platinum contact(s) and each edge of the cuff. For each experiment the electrode was placed such that the distal edge of the nerve cuff was at the common peroneal branch point as shown in Fig. 2. Current-controlled 40-kHz sinusoidal blocking waveforms were delivered through the blocking electrode using a Keithley 6221 waveform generator (Keithley Instruments, Cleveland, OH) for each trial.
The effect of bipolar separation distance on the onset response was tested by applying different distal blocking electrodes to the nerve of each rat and measuring the response to the HFAC. Four bipolar separation distances were tested in each animal: 1.0, 2.0, 3.0, and 4.0 mm were tested in two animals, and 0.5, 1.0, 2.0, and 4.0 mm were tested in three animals. The order in which the electrodes were tested was block randomized for each animal, in three statistical blocks (one electrode of each geometry was tested in each statistical block). After each electrode placement, the following five-step procedure was used.
-
Step 1)
A trial was performed to calculate the block threshold (the minimal blocking current amplitude that will result in a complete nerve block [4], [6], [11], [14], [15]) using a procedure described previously [14], [15]. Each trial consisted of a period of approximately 5 s of 1 Hz proximal stimulation, followed by a period of proximal stimulation combined with a distal HFAC waveform. For each trial the HFAC amplitude was initially 9.0 mApeak. After approximately 5 s (to allow for the onset response to fully subside), the amplitude of the high-frequency waveform was decremented by 0.1 mA per second until muscle twitches were detected. The lowest amplitude at which no muscle twitches were present was determined to be the block threshold. For trials in which 9.0 mA was not sufficient to block the nerve, the trial was repeated starting at 11.0 mA. For one trial, 11.0 mA was not sufficient to block the nerve, and the block threshold was measured starting at 13.0 mA.
-
Step 2)
A high-frequency onset trial was executed. For these trials, a single supramaximal 20 μs pulse was delivered through the proximal stimulation electrode, followed by four 5-s bursts of HFAC delivered to the nerve through the distal blocking electrode. Each of these bursts is followed by a 3-s interburst interval with no current delivery. The amplitude of each burst of HFAC was randomized for each trial to one of the following: block threshold amplitude (as calculated from the previous trial), 125% of block threshold amplitude, 150% of block threshold amplitude or 200% of block threshold amplitude.
-
Step 3)
A 2-min wait was imposed to minimize any effect of nerve accommodation to the HFAC on the onset response.
-
Step 4)
The block threshold was measured again using the procedure described above.
-
Step 5)
A second high–frequency onset trial was performed by the procedure described above, using the block threshold amplitude calculated from the previous trial (step 4).
After step 5 was completed, the electrode was removed and replaced with a different electrode per the randomization scheme. Steps 1–5 were executed for the new geometry, and so on.
This protocol resulted in a total of 24 randomized individual observations for each electrode size tested in each animal (each electrode was tested in three statistical blocks per animal, each with a once repeated observation, at four different amplitudes). This resulted in a total of 480 measurements from which the onset response was analyzed as a function of the electrode contact separation (Note: due to a technical malfunction during the experiment, one measurement could not be made, and one of the repeated series of observations was omitted, resulting in 475 actual recorded observations).
C. Data Analysis and Statistics
Three different measures were used to assess the magnitude of the onset response for each HFAC burst: the peak force of the muscle response [4], the integral of the muscle force over the period of the burst [4] and the time required for the onset response to decay to 5% of its peak value from the time of peak. These measures are shown schematically in Fig. 1. The peak force is a measure of Phase I neural activity and is an indicator of the degree of intensity of the initial neural volley that occurs when the HFAC is first applied [19]. The force integral and decay time are measures of Phase II of the onset response. The force integral is an indicator of the intensity of the Phase II firing and was calculated by integrating the force over the entire period of the HFAC burst. The decay time is an indicator of the duration of Phase II neural firing and was calculated to be the time required for the muscle force to drop to 5% of its peak value from the time of peak. For trials in which the muscle force did not decay to 5% within the burst interval, the decay time was estimated by extrapolating the muscle force which was recorded over the burst interval using a linear regression estimate. The regression estimate was made using the last second of muscle force data.
The outcome measures from the study were continuous data from the factorial experimental design. We examined the effects of the two studied factors and their interaction: the bipolar separation and the waveform amplitude. Two types of statistical models were considered here: a standard linear model and a linear random-effects model (LREM), where a random effect was included to take the variability of subjects into account [22]. LREM is a generalization of the standard linear model, permitting the data to exhibit correlation and nonconstant variability. The LREM, hence, provides flexibility of modeling not only the means of our data (as in standard linear model) but also their variance and covariance.
We firstly modeled the untransformed data and then examined the models using appropriate statistical diagnostic tools. The fitted models proved inappropriate because of the skewness of the outcome distributions. Thus, the outcomes were transformed using a log-transformation to make them normally distributed. The regression models based on the log-transformed outcomes were suitable based on the further statistical diagnostics. Akaike information criterion (AIC) was used to compare the different fitted models. LREMs were uniformly better than the standard linear models for all outcomes.
Therefore, each of our final statistical models for this study were LREMs for log-transformed outcomes. ANOVA F tests [23] were constructed based on the LREMs to test the factor effects at the 0.05 significance level.
III. Results
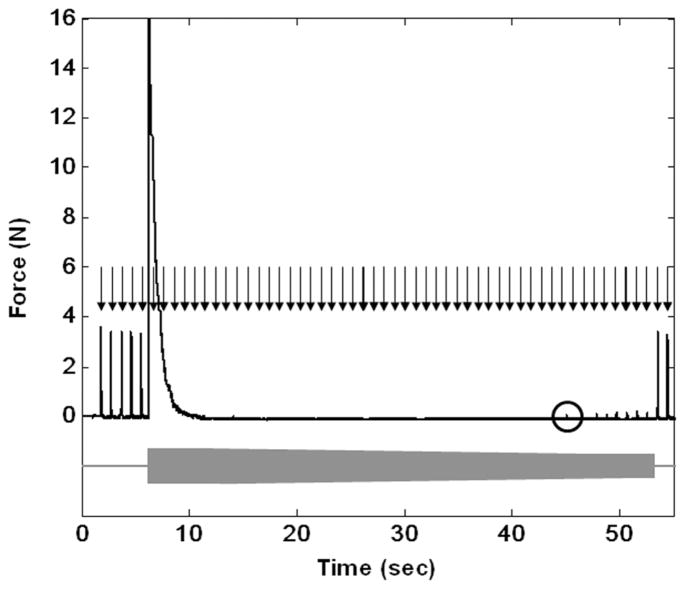
Complete conduction block was readily achieved in each of the animals tested. Fig. 3 shows a typical 40 kHz HFAC block threshold trial for a bipolar electrode with a contact separation of 1.0 mm. The figure depicts a trial in which the block threshold was measured by decreasing the blocking current amplitude until partial nerve conduction block allows for small amplitude muscle twitches to be generated from the proximal stimulation. The absence of muscle twitches during proximal stimulation pulse delivery indicates complete conduction block, and small amplitude muscle twitches indicate partial conduction block. The circle in Fig. 3 highlights the first muscle twitch which results from a partially conducting nerve.
Fig. 3.
Block threshold trial for a bipolar electrode with contact separation of 1.0 mm. Supramaximal twitches are delivered at ~1 Hz through the proximal electrode as designated by the black arrows. As indicated by the grey bar, the HFAC is turned on at approximately 6.5 s with an amplitude of 9.0 mA and is decremented in 0.1 mA steps until conduction block is no longer complete. The circle designates the first twitch conducted by a partially conducting nerve. Full conduction is restored by the following proximal stimulation pulse.
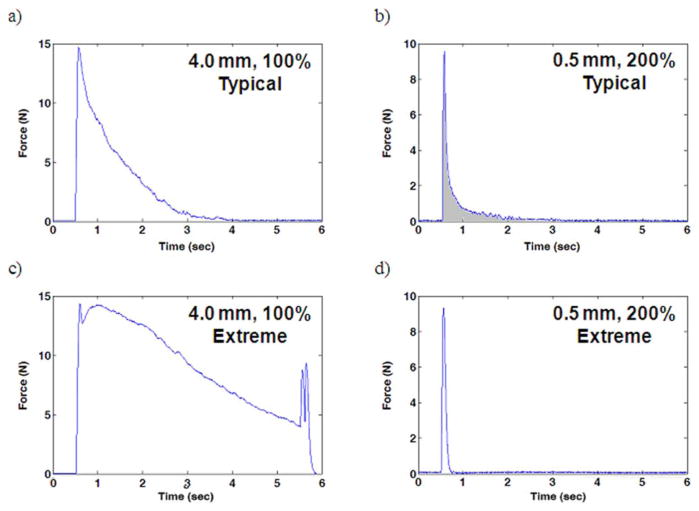
The effect of bipolar separation distance on Phase I and Phase II of the onset response was tested in five rats at four different amplitudes. Fig. 4 depicts various onset responses to 5-s bursts of HFAC recorded during high-frequency onset response trials. Trials in which low amplitudes of HFAC (100% of block threshold) were delivered through 4.0 mm electrodes tended to produce the most onset firing. Trials in which high amplitudes of HFAC (200% of block threshold) were delivered through 0.5 mm electrodes tended to produce the least onset firing. Fig. 4(a) and (b) shows typical onset response trials for each of these experimental conditions, respectively. Fig. 4(c) and (d) shows extreme onset response trials for each of these experimental conditions, respectively, and are included to show the magnitude range for the onset responses observed in these experiments. Fig. 4(c) shows a trial with a large onset response during which Phase II does not subside within the duration of the HFAC burst. Fig. 4(d) shows a trial with minimal onset response for which there is no appreciable Phase II firing. The large muscle twitch occurring at 5.5 s in the trial displayed in Fig. 4(c) coincides with the cessation of the HFAC burst. This “off response” has been described elsewhere [5] and occurred occasionally.
Fig. 4.
Muscle force recordings from bipolar high-frequency onset burst trials. (a) Typical onset response for an electrode with a bipolar separation distance of 4.0 mm at 100% block threshold. (b) Typical onset response for an electrode with a bipolar separation distance of 0.5 mm at 200% block threshold. (c) Extreme onset response for an electrode with a bipolar separation distance of 4.0 mm at 100% block threshold. (d) Extreme onset response for an electrode with a bipolar separation distance of 0.5 mm at 200% block threshold.
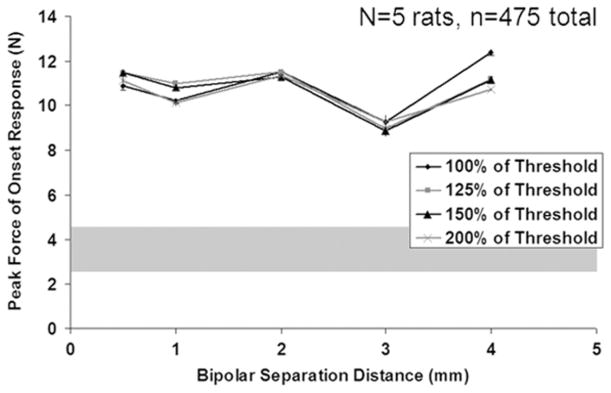
The degree of Phase I firing was assessed by measuring the peak force of the onset response for each high-frequency onset burst trial. Fig. 5 shows the peak force as a function of bipolar separation distance for each of the four amplitudes tested. These data represent the mean across all animals, with error bars representing the standard error (a representation of the accuracy of the mean estimate). The approximate experimental range for the maximal single twitch force is shown by the grey shaded region. The peak force is similar for all bipolar separation distances tested, with a slight reduction in force when using a blocking electrode with a bipolar separation of 3.0 mm (15% difference between the 3.0 mm mean and population mean). Statistical analysis shows that the bipolar separation distance does have a significant effect on the peak force (p = 0.0015). Statistical analysis shows that the blocking amplitude does not have a significant effect on the peak force of the onset response (p = 0.97). The peak force for each combination of HFAC amplitude and bipolar separation distance is substantially higher than a single supramaximal twitch elicited by proximal stimulation; the peak force was an average of 3.5 times the force of the supramaximal twitch (with a range of approximately 1.0–8.0 times the twitch force) that preceded each high-frequency onset trial across all trials. This is consistent with previously published data regarding peak force of the onset response [4].
Fig. 5.
Peak force with bipolar separation distance and HFAC amplitude for the five rats (475 observations in total). Error bars represent the standard error of the mean (Note: standard deviation is √24 =~ 4.9 times larger than the SEM). The grey shaded region represents the approximate range of experimentally measured proximal twitch force.
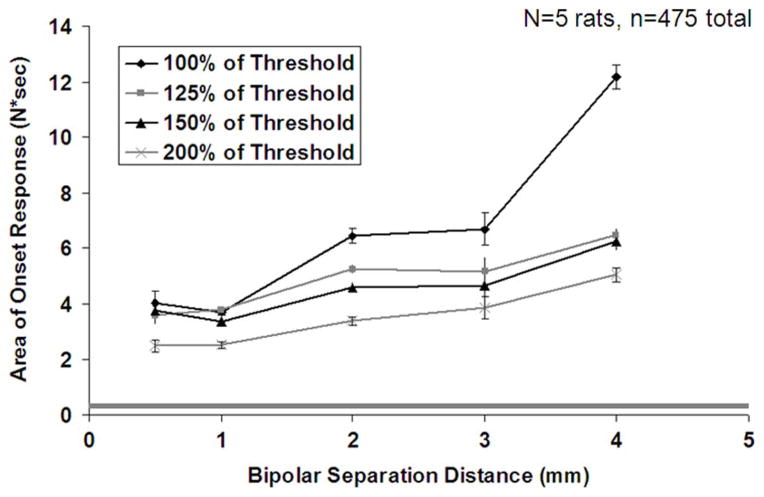
The degree of Phase II firing was assessed using two measures: the integral of the muscle force over the period of a 5-s HFAC burst and the time required for the onset response to decay to 5% of its peak value. Fig. 6 shows the integral of the muscle force as a function of bipolar separation distance for each of the four amplitudes tested. The error bars represent the standard error (a representation of the accuracy of the mean estimate). These data show a general increase in the integral of the onset response with increasing bipolar separation distance and a decrease with increasing HFAC amplitude. Statistical analysis shows that both bipolar separation distance and amplitude have a significant effect on the onset integral (p = 0.0003 and p = 0.0025, respectively). The integral of the onset response for the 4.0 mm trials with a HFAC amplitude equal to block threshold is substantially greater than any other bipolar separation and amplitude combination.
Fig. 6.
Integral of onset response with bipolar separation distance and HFAC amplitude for the five rats (475 observations in total). Error bars represent the standard error of the mean (Note: standard deviation is √24 =~ 4.9 times larger than the SEM). The grey shaded region represents the approximate range of the integral of experimentally measured proximal twitch force.
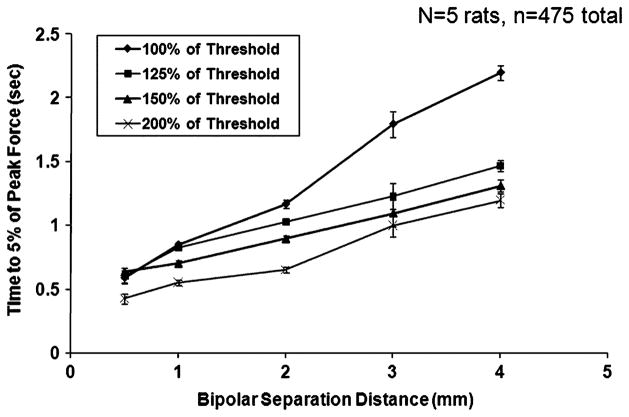
Fig. 7 shows the mean time required for the onset response to decay to 5% of its peak value as a function of bipolar separation distance for each of the four amplitudes tested. The error bars represent the standard error (a representation of the accuracy of the mean estimate). These data show a general increase in the decay time for the onset response with bipolar separation distance and a decrease with HFAC amplitude. Statistical analysis confirms that both bipolar separation distance and amplitude have a significant effect on the decay time (p < 10−4 and p < 10−4, respectively). The combination of a 0.5 mm bipolar separation distance and an amplitude of 200% of block threshold resulted in the shortest mean decay time. The combination of a 4.0 mm bipolar separation distance and an amplitude of 100% of block threshold resulted in the longest mean decay time. There were five trials in which the muscle force did not decay to 5% within the 5-s interval. Each of these trials occurred when using an electrode with a 4.0 mm separation distance. Three of these trials occurred when a HFAC amplitude of 100% of block threshold was applied, one occurred with an amplitude of 150% of block threshold, and the other occurred with an amplitude of 200% of block threshold.
Fig. 7.
Time for muscle force to decay to 5% of peak value with bipolar separation distance and HFAC amplitude for the five rats (475 observations in total). Error bars represent the standard error of the mean (Note: standard deviation is √24 =~ 4.9 times larger than the SEM).
IV. Discussion
The results presented in this study support the hypothesis that there are two distinct phases of the HFAC onset response. In this study, Phase I was characterized by a brief high magnitude muscle twitch (~3.5 times the suprathreshold twitch force in this study) and was not found to be substantially affected by nerve cuff bipolar separation distance or HFAC amplitude. Phase II of the onset response was characterized by a lower magnitude force that typically decayed over a period of 5 s or less, and was highly dependent on the bipolar separation distance and HFAC amplitude. Reducing the magnitude and duration of the onset response is important for moving HFAC nerve block into human applications. The results of this study show that a proper selection of electrode geometry and waveform amplitude can substantially reduce the duration and magnitude of Phase II of the onset response. In some cases, it was possible to eliminate the Phase II firing with the proper selection of these parameters.
The bipolar separation distance was shown to have a substantial effect on Phase II of the onset response, exhibiting a monotonic increase in onset integral and decay time with bipolar separation distance at all four amplitudes tested. For example, at a blocking amplitude of 100% of block threshold the mean onset integral varied by a factor of 3.0 over the entire range of bipolar separation distances. The mean time for the force to decay to 5% of its initial value varied by a factor of 3.8 over this same range. The amplitude of the HFAC waveform also had a substantial effect on Phase II of the onset response for the bipolar experiments. There was a monotonic decrease in onset integral and decay time with increases in amplitude for all bipolar separation distances with the possible exception of 0.5 mm and 1.0 mm, where the onset response is very similar for the lowest three amplitudes. This trend is consistent with previously published data showing smaller onset responses with increases in amplitude [2], [4], [8], [9], [11]. This is the first study which has examined the effect of HFAC amplitude on the onset response using multiples of the block threshold instead of fixed waveform voltages or currents. These data suggest that the onset response does trend with multiples of block threshold. Over the entire range of bipolar separation distance and amplitudes tested, the onset integral and decay time varied by a factor of 4.9 and 5.2, respectively.
The data presented in this study show that the selection of the blocking electrode geometry is important to inducing a nerve conduction block with a brief onset response. For the bipolar cuffs used in this study, the onset response was minimized for electrodes with a bipolar separation distance of 0.5 mm over the range of separation distances tested. The data also suggest that to some degree a poor electrode configuration which produces an intense onset response can be compensated for with an increase in amplitude.
In addition to minimizing the onset response, minimizing the total charge needed to produce block is also an important design criterion. In a previously study published by our laboratory, electrode geometry was found to have a significant effect on HFAC block threshold. For bipolar electrodes, block threshold was shown both experimentally and in single fiber simulation to exhibit a U-shaped trend with bipolar separation distance with the minimum thresholds occurring for separation distances of 1.0–2.0 mm [15]. Typical thresholds ranged from 5.0 to 7.5 mA depending on the electrode configuration [15]. The data in this study show that the onset response does not trend in this same way, suggesting that some electrode configurations (e.g., a bipolar separation of 0.5 mm) are better for producing a block with a small onset response and others (e.g., a bipolar separation of 2.0 mm) are better for producing a block with a low block threshold. The weighting given to the magnitude of onset response versus total charge injection in the design process will likely depend on the clinical application. Factors such as the clinical tolerance for onset response firing and the power budget for an implantable pulse generator would likely be important to consider since the amount of power required for HFAC block is significantly larger than that required for typical neurostimulation.
The frequency of the blocking waveform has also been shown to significantly impact the magnitude of the onset response [2], [4], [10]–[13]. Higher frequency blocking waveforms result in a lesser onset response than lower frequency waveforms. However, high-frequency blocking waveforms require larger current amplitudes to achieve block than low frequency blocking waveforms. Since the onset response is transient, a transitioning paradigm may make it possible to take advantage of both ends of the frequency, amplitude and electrode spectrums. For example, a blocking therapy which starts with a high-frequency, high amplitude waveform that is delivered through a bipolar electrode with a 0.5 mm contact would produce little onset response, but would not be optimal for minimizing power consumption. This same therapy could ramp down the frequency and amplitude, and could possibly even transition current delivery to contacts with a spacing of 2.0 mm to reduce long-term power consumption.
The experimental results presented also provide some significant insight into why Phase I and Phase II of the onset response occur, why Phase II, but not Phase I, is strongly affected by electrode geometry and waveform, and why Phase II typically diminishes quickly. The biophysical nature of Phase I of the onset response is better understood than that of Phase II. A previously published simulation study suggests that Phase I of the onset response is a series of action potentials that occurs during the transition from a hyperpolarized to a depolarized state which occurs in HFAC block [18]. The very large muscle twitches seen in whole nerve preparations are consistent with this hypothesis. A study by Karu, et al. on rapid n-let stimulation of peripheral nerve demonstrated that multiple rapid efferent action potentials result in a large supra-maximal muscle twitch similar to that which characterizes Phase I [19]. There are no published studies of blocking a nerve or an axon using HFAC which have not resulted in Phase I firing, and modification of neither electrode configuration nor waveform amplitude prevented its occurrence in this study.
While Phase II of the onset response is less well understood, the experimental results provide some significant insight that allow for a hypothesis as to why Phase II is strongly affected by electrode geometry and amplitude and why its intensity typically diminishes with time. We hypothesize that the neural activation which occurs during Phase II is initiated at regions of the nerve which experience a field intensity which is subthreshold for nerve block, and that the temporal decay of the firing intensity may result from ion accumulation in the space surrounding the axons.
It is well established that when current is delivered through a nerve cuff electrode, a large field intensity is produced at the site of the electrode contacts, and a lesser, but significant, “virtual electrode” field intensity occurs at the edges of the cuff [24]. The geometry of a nerve cuff electrode and the amplitude of HFAC would affect the intensity of these virtual electrode fields. Simulations by Bhadra et al. and single fiber recordings performed by Bowman and McNeal have demonstrated that neurons subjected to HFAC with an amplitude that is subthreshold for block undergo rapid firing [8], [18]. Each of these studies has also shown that the firing rate decreases with an increase in subthreshold HFAC amplitude (above 50% of block threshold) [8], [18]. The intensity of these subthreshold fields would increase with an increase in blocking current amplitude. The data presented in this study show a decrease in Phase II firing with an increase in HFAC amplitude, which is consistent with this hypothesis. The effect of electrode geometry on the magnitude of subthreshold field intensity could likely be better elucidated with a finite element modeling study. However, definite changes in Phase II firing with electrode geometry have been demonstrated, suggesting that subthreshold fields could play a role in the trends shown.
We hypothesize that the temporal decay in Phase II firing occurs due to a slow membrane depolarization for axons subjected to subthreshold field intensities, and that this slow depolarization is caused by an accumulation of potassium in the extracellular space. In a single fiber study, Bowman and McNeal showed that axons which are subjected to HFAC that is subthreshold for block exhibit a decay from high firing rates (200–500 Hz) to zero over the course of 5–10 s [8]. In a modeling study, Bhadra, et al. showed that decreased firing rates which occur with increases in HFAC amplitude are associated with a membrane depolarization [18].
Extracellular potassium accumulation is known to cause membrane depolarization [25], and has been associated with conduction block in CNS neurons [26], [27]. It has been shown that extracellular potassium accumulates in peripheral nerves when they undergo high rates of firing. [28], and we hypothesize that this accumulation results in the decay in firing rate seen in Phase II of the onset response.
It is of note that basic physiological studies of nerve conduction and fiber type functionality sometimes employ electrical nerve blocking techniques (namely direct current block) to block the activity of a particular fiber population. Since direct current block is known to cause damage to nerve fibers [29], HFAC might serve as an attractive alternative nerve blocking technique for these studies. Since it is sometimes desirable to implement such a conduction block with minimal firing [30], the results of this study could be used to guide the selection of an electrode geometry for this type of study.
V. Conclusion
We have shown through randomized and repeated experiments that the contact separation distance of a bipolar nerve cuff electrode has an effect on the onset firing response to a HFAC blocking waveform. We also present data confirming trends shown by others that demonstrate a decrease in the onset response with an increase in HFAC amplitude, and for the first time present this type of analysis using multiples of the block threshold. These data suggests that there are two distinct phases to the onset response. Phase I was characterized by a brief high magnitude muscle twitch and was not found to be substantially affected by nerve cuff electrode geometry or HFAC amplitude. Phase II of the onset response was characterized by a lower magnitude force that typically decayed over a period of 5 s or less, and was effected by the bipolar separation distance and HFAC amplitude. The data suggest that Phase II onset firing could be the result of subthreshold fields remote to the electrode contacts that cause a decaying firing response. These data have implications for the selection of a nerve blocking electrode geometry for clinical applications or basic neurophysiology research. For example, conduction block with a short duration onset response could be useful in implementing a combined thermal or direct current onset-mitigating strategy [31], [32].
Acknowledgments
This work was supported by the National Institute of Biomedical Imaging and Bioengineering under Grant R01-EB-002091.
Biographies

D. Michael Ackermann, Jr. received the B.S. degree in biomedical engineering from Vanderbilt University, Nashville, TN, in 2004, and the M.S. and Ph.D. degrees in biomedical engineering from Case Western Reserve University, Cleveland, OH, in 2007 and 2010, respectively. He is currently a fellow at the Biodesign Institute at Stanford University, Palo Alto, CA.
His research interests include neurostimulation, nerve conduction block, implantable device design, computational neuroscience and neuroprosthetics for the restoration of motor control and other applications.

Niloy Bhadra (M’05) received the M.B.B.S. degree and the M.S. degree in orthopaedic surgery from Calcutta University, India, in 1982 and 1985, respectively. He became a Fellow of the Royal College of Surgeons, Edinburgh, U.K., in 1989. He received the M.S. and Ph.D. degrees in biomedical engineering from Case Western Reserve University, Cleveland, OH, in 2000 and 2005, respectively.
He is currently Research Assistant Professor in the Department of Biomedical Engineering, Case Western Reserve University. His research interests include rehabilitation engineering, functional electrical stimulation, neural prostheses, and nerve conduction block.

Emily L. Foldes received the B.S. degree in electrical engineering and biomedical engineering from Washington University, St. Louis, MO, in 2004, and the M.S. degree in biomedical engineering from Case Western Reserve University, Cleveland, OH, in 2007.
She is currently working as a Biomedical Engineer in the Department of Biomedical Engineering at Case Western Reserve University, Cleveland, OH, and is part of the Cleveland Functional Electrical Stimulation Center. Her research interests include using electrical stimulation and implantable medical devices for clinical applications.

Xiao-Feng Wang received the M.S. degree in applied mathematics from Huazhong University of Science and Technology, Hubei, China, and the Ph.D. degree in statistics from Case Western Reserve University, Cleveland, OH.
He is Assistant Staff of Biostatistics in the Department of Quantitative Health Sciences at the Cleveland Clinic and Assistant Professor of Medicine and Biostatistics at the Case Western Reserve University School of Medicine. His main specialties are in non-parametric statistics, measurement error models, and statistical data mining.
Dr. Wang is an Elect Member of the International Statistical Institute.

Kevin L. Kilgore received the B.S. degree in biomedical engineering from the University of Iowa, Iowa City, in 1983, and the M.S. and Ph.D. degrees in biomedical engineering from Case Western Reserve University, Cleveland, OH, in 1987 and 1991, respectively.
He is currently Functional Electrical Stimulation (FES) Program Manager in the Department of Orthopaedics at MetroHealth Medical Center, Biomedical Engineer in the Research Service of the Louis Stokes Cleveland Veterans Affairs Medical Center, and Adjunct Assistant Professor in the Department of Biomedical Engineering, Case Western Reserve University. He is an Associate Director in the Cleveland FES Center. His research interests are in the clinical applications of functional electrical stimulation to provide hand and arm function for individuals with paralysis, and in the application of electrical currents to control unwanted neural activity.
Contributor Information
D. Michael Ackermann, Jr., Email: dma18@case.edu, Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH 44106 USA, and also with the Department of Orthopedics MetroHealth Medical Center, Cleveland, OH 44109 USA, and also with the Cleveland FES Center, Cleveland, OH 44109 USA
Niloy Bhadra, Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH 44106 USA.
Emily L. Foldes, Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH 44106 USA
Xiao-Feng Wang, Department of Quantitative Health Sciences, Cleveland Clinic Foundation, Cleveland, OH 44195 USA.
Kevin L. Kilgore, Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH 44106 USA, and also with the Department of Orthopedics, MetroHealth Medical Center, Cleveland, OH 44109 USA, and also with the Louis Stokes Veterans Affairs Medical Center, Cleveland, OH 44106 USA
References
- 1.Boger A, Bhadra N, Gustafson KJ. Bladder voiding by combined high frequency electrical pudendal nerve block and sacral root stimulation. Neurourol Urodynamics. 2008;27(5):435. doi: 10.1002/nau.20538. [DOI] [PubMed] [Google Scholar]
- 2.Gaunt R, Prochazka A. Transcutaneously coupled, high-frequency electrical stimulation of the pudendal nerve blocks external urethral sphincter contractions. Neurorehabil Neural Repair. 2009;23:615. doi: 10.1177/1545968308328723. [DOI] [PubMed] [Google Scholar]
- 3.Peckham H, Knutson J. Functional electrical stimulation for neuromuscular applications. Annu Rev Biomed Eng. 2005;7(1):327. doi: 10.1146/annurev.bioeng.6.040803.140103. [DOI] [PubMed] [Google Scholar]
- 4.Bhadra N, Kilgore KL. High-frequency electrical conduction block of mammalian peripheral motor nerve. Muscle Nerve. 2005;32(6):782. doi: 10.1002/mus.20428. [DOI] [PubMed] [Google Scholar]
- 5.Kilgore K, Bhadra N. Nerve conduction block utilizing high-frequency alternating current. Med Biol Eng Comput. 2004;42(3):394–406. doi: 10.1007/BF02344716. [DOI] [PubMed] [Google Scholar]
- 6.Williamson RP, Andrews BJ. Localized electrical nerve blocking. IEEE Tran Biomed Eng. 2005 Mar;52(3):362–370. doi: 10.1109/TBME.2004.842790. [DOI] [PubMed] [Google Scholar]
- 7.Woo MY, Campbell B. Asynchronous firing and block of peripheral nerve conduction by 20 kC alternating current. Bull Los Angel Neuro Soc. 1964;29:87. [PubMed] [Google Scholar]
- 8.Bowman B, McNeal D. Response of single alpha motorneurons to high-frequency pulse trains. Appl Neurophysiol. 1986;49:121–138. doi: 10.1159/000100137. [DOI] [PubMed] [Google Scholar]
- 9.Rosenblueth A, Reboul J. The blocking and deblocking effects of alternating currents on nerve. Am J Physiol. 1939 Jan 31;125(2):251–264. [Google Scholar]
- 10.Tai C, Roppolo JR, de Groat WC. Block of external urethral sphincter contraction by high frequency electrical stimulation of pudendal nerve. J Urol. 2004;172(5)(pt. 1):2069–2072. doi: 10.1097/01.ju.0000140709.71932.f0. [DOI] [PubMed] [Google Scholar]
- 11.Bhadra N, et al. High frequency electrical conduction block of the pudendal nerve. J Neural Eng. 2006;3(2):180. doi: 10.1088/1741-2560/3/2/012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miles JD, et al. Effects of ramped amplitude waveforms on the onset response of high-frequency mammalian nerve block. J Neural Eng. 2007;4(4):390. doi: 10.1088/1741-2560/4/4/005. [DOI] [PubMed] [Google Scholar]
- 13.Joseph L, Haeffele BD, Butera RJ. Conduction block induced by high frequency AC stimulation in unmyelinated nerves. Proc Annu Int Conf IEEE Eng Med Biol Soc. 2007 Aug;2007:1719–1722. doi: 10.1109/IEMBS.2007.4352641. [DOI] [PubMed] [Google Scholar]
- 14.Ackermann D, et al. Conduction block of peripheral nerve using high frequency alternating currents delivered through an intrafascicular electrode. Muscle Nerve. 2009 doi: 10.1002/mus.21496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ackermann D, et al. Effect of bipolar cuff electrode design on block thresholds in high frequency electrical neural conduction block. IEEE Trans Neural Syst Rehabil Eng. 2009 Oct;17(5):469–477. doi: 10.1109/TNSRE.2009.2034069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foldes E, et al. Counted cycles method to quantify the onset activity in high-frequency peripheral nerve block. Conf Proc Annu Int Conf IEEE Eng Med Biol Soc. 2009 Sep;:614–617. doi: 10.1109/IEMBS.2009.5332758. [DOI] [PubMed] [Google Scholar]
- 17.Tai Simulation of nerve block by high-frequency sinusoidal electrical current based on the Hodgkin-Huxley model. IEEE Trans Neural Syst Rehabil Eng. 2005 Sep;13(3):415–422. doi: 10.1109/TNSRE.2005.847356. [DOI] [PubMed] [Google Scholar]
- 18.Bhadra N, et al. Simulation of high-frequency sinusoidal electrical block of mammalian myelinated axons. J Comput Neurosci. 2007;22:313–326. doi: 10.1007/s10827-006-0015-5. [DOI] [PubMed] [Google Scholar]
- 19.Karu ZZ, Durfee WK, Barzilai AM. Reducing muscle fatigue in FES applications by stimulating with N-let pulse trains. IEEE Trans Biomed Eng. 1995 Aug;42, 8(8):809–817. doi: 10.1109/10.398642. [DOI] [PubMed] [Google Scholar]
- 20.Ackermann D, et al. Electrode design for high frequency block: Effect of bipolar separation on block thresholds and onset activity. Conf Proc Annu Int Conf IEEE Eng Med Biol Soc. 2009 Sep;:654–657. doi: 10.1109/IEMBS.2009.5332738. [DOI] [PubMed] [Google Scholar]
- 21.Schmalbruch H. Fiber composition of the rat sciatic nerve. Anatomical Rec. 1986;215(1):71. doi: 10.1002/ar.1092150111. [DOI] [PubMed] [Google Scholar]
- 22.Davis C. Statistical Methods for the Analysis of Repeated Measurements. New York: Springer; 2002. [Google Scholar]
- 23.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer; 2000. [Google Scholar]
- 24.Sweeney JD, Mortimer JT. An asymmetric two electrode CUF for generation of unidirectionally propagated action potentials. IEEE Trans Biomed Eng. 1986 Jun;33(6):541–549. doi: 10.1109/TBME.1986.325818. [DOI] [PubMed] [Google Scholar]
- 25.Frankenhaeuser B. The after-effects of impulses in the giant nerve fibres of loligo. J Physiol (Paris) 1956;131(2):341. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen ADD. High frequency stimulation can block axonal conduction. Exp Neurol. 2009;220(1):57. doi: 10.1016/j.expneurol.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellinger SMG, Steinmetz P. Submyelin potassium accumulation may functionally block subsets of local axons during deep brain stimulation: A modeling study. J Neural Eng. 2008;5(3):263. doi: 10.1088/1741-2560/5/3/001. [DOI] [PubMed] [Google Scholar]
- 28.Hoppe D, et al. Characteristics of activity-dependent potassium accumulation in mammalian peripheral nerve in vitro. Brain Res. 1991;552:106–112. doi: 10.1016/0006-8993(91)90666-j. [DOI] [PubMed] [Google Scholar]
- 29.Merrill D. Electrical stimulation of excitable tissue: Design of efficacious and safe protocols. J Neurosci Methods. 2005;141(2):171. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 30.Petruska JC, Hubscher CH, Johnson RD. Anodally focused polarization of peripheral nerve allows discrimination of myelinated and unmyelinated fiber input to brainstem nuclei. Exp Brain Res. 1998;121(4):379. doi: 10.1007/s002210050472. [DOI] [PubMed] [Google Scholar]
- 31.Ackermann D, et al. Nerve block using combined thermoelectric cooling and high frequency alternating current: Electrical block without onset response. J Neurosci Methods. 2001;193(1):72–76. doi: 10.1016/j.jneumeth.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ackermann D, et al. Peripheral nerve block using combined high frequency alternating and direct currents for mitigation of onset firing. Med Biol Eng Comput. 2001 doi: 10.1007/s11517-010-0679-x. to be published. [DOI] [PMC free article] [PubMed] [Google Scholar]