Abstract
The availability of lumbar interbody cages has fuelled renewed interest in interbody fusion. Despite this, there is no consensus regarding the best non-invasive method for evaluation of interbody fusion, especially where cages have been used. The purpose of this study was to determine whether high-quality thin-slice (1- to 3-mm) computed tomography (CT) scans allow proper evaluation of interbody fusion through titanium cages. Patients undergoing lumbar interbody fusion were prospectively evaluated with CT scan and plain radiographs 6 months following surgery. These images were blindly and independently evaluated by a consultant radiologist and a spine research fellow, for bridging bony trabeculation both through and surrounding the cages as well as for changes at the cage endplate interface. Fifty-three patients (156 cages) undergoing posterior lumbar interbody fusion using titanium interbody cages were evaluated. Posterior elements were used to pack the cages and no graft was packed outside the cages. The outcome data were analysed using the Kappa co-efficient and chi-squared analysis. On CT scan, both observers noted bridging trabeculation in 95% of the cages (Kappa 0.85), while on radiographs this was present in only 4% (Kappa 0.74). Both observers also identified bridging trabeculation surrounding the cages on CT scan in 90% of cages (Kappa 0.82), while on the radiographs this was 8% (Kappa 0.86). Radiographs also failed to demonstrate all the loose cages. The results of the study show that high-quality CT scans show images suggesting bridging bony trabeculae following the use of titanium interbody cages. They also appear to show consistent bone outside the cages in spite of no bone graft having been used, and they appear to be better than plain radiographs in the early detection of cage loosening.
Keywords: Lumbar interbody fusion, Plain radiographs, CT
Introduction
The use of cages to help achieve interbody fusion in the treatment of the painful back has been increasing in popularity [4, 10, 18, 27, 30]. The cages are usually packed with autograft, allograft or bone substitute, and can be introduced either through an anterior [17, 18] or a posterior approach [4, 14]. They can be stand alone or can be supplemented with posterior instrumentation [4, 17] for additional stability, and can be made of metal, usually titanium [18, 27] or a carbon-fiber composite (PEEK, PEKEKK) [5, 11]. Structurally, they can be classified as horizontal cylinders, vertical rings, or open boxes [30]. All cages have one property in common: they all have openings through which the bone graft or bone substitute can achieve osseous integration with adjacent endplates. This should result in production of continuous crossing bony trabeculation between adjacent endplates through the cage, which should consequently realize the ultimate aim of a successful interbody fusion.
Review of the literature shows that there are no universally accepted radiological criteria for assessing interbody fusion, even when only autograft has been used. The use of interbody cages with or without posterior instrumentation has complicated matters even further.
Stauffer and Coventry [29], in their radiological evaluation of anterior lumbar interbody fusion using plain radiographs and tomograms, set as the criteria suggestive of solid fusion a continuous bony trabeculation traversing the grafted level and the adjacent vertebral bodies, with no evidence of motion on bending films. They also observed that in most patients in whom pseudarthrosis developed, this was obvious within 6 to 9 months of surgery.
Pearcy and Borrough [26] found that using the appearance of bony trabeculation crossing the fusion mass and remodelling of the anterior edges of the anterior endplate on plain radiographs overestimated fusion. Using biplanar radiographic measurements they were able to demonstrate intersegmental movement in five out of ten patients with conventional evidence of fusion, resulting in three of these five being classed as having a non-union.
Greenough et al. [13] also used presence of crossing bony trabeculation as well as movement of less than 1° on flexion/extension radiographs as their definition of anterior interbody fusion with the use of iliac autograft, using computed tomographic (CT) scans in cases of doubt. However, CT criteria indicative of fusion were not defined.
Blumenthal and Gill [1] found, on routine removal of posterior instrumentation at 9 months following interbody and posterolateral fusion, that plain radiographs underestimated the fusion rate in 20% of cases (one in five). Brodsky et al. [6] also reported the difficulty in radiologically assessing spinal fusion, though this was in relation to posterior fusion. They did note that CT scans, which were not done specifically for the evaluation of the fusion, were the least inaccurate.
Larsen et al. [21] evaluated the use of isotope bone scan to assess fusion, and found its predictive value to be negligible. Even though this was for posterolateral grafting, it would be reasonable to extrapolate that it would have the same problem for interbody fusion.
Rothman et al. [28] introduced the concept of curved coronal reformations using CT, which enabled considerably better visualisation of the lumbar spine. Lang et al. [20] found that interpretation of sagittal and curved coronal multiplanar reconstruction (MPR) was more reliable than any other imaging method applied to the detection of spinal fusion. In a series of 30 patients with posterior lumbar fusion, they were able to identify pseudarthrosis in four patients using the above modality, all of which were confirmed at surgery. Conventional radiographs yielded the lowest incidence of pseudarthrosis. Although their study was undertaken on patients with posterior fusion, the usefulness of the technique was beyond doubt.
Laasonen and Soini [19], using CT with 6-mm slice thickness and selective sagittal reconstruction also felt it was the best method to evaluate posterolateral fusion. Brodsky et al. [6], Herzog and Marcotte [15], and Leuvén [22] felt that high-resolution, thin-section coronal and sagittal reformatted images were likely to be the most useful modalities in the evaluation of spinal fusion. Larsen et al. [21] were, however, unable to assign a predictive value to CT, but it should be noted that they were using overlapping 5-mm-thick sections at 3-mm intervals to assess posterolateral fusion. It would also appear that the posterior pedicular instrumentation in their series was of stainless steel, thereby producing significant artefact. Zdeblick [24] recently noted that fine-section sagittal and coronal reconstruction images on CT should enable visualization of crossing trabecular bone through the second and third generation interbody devices.
The senior author uses titanium interbody cages (Ogival, Stryker, UK) with titanium transpedicular instrumentation (Diapason, Stryker, UK) in the management of selected patients with chronic disabling low back pain. It was postulated that high-quality thin-slice 1- to 3-mm CT images should allow proper evaluation of the fusion status through the titanium cages, which was not possible with routine plain radiographs. Therefore, to test this hypothesis, a prospective study was performed on patients undergoing lumbar interbody fusion, in which they were evaluated prospectively at 6 months post surgery with both CT and plain radiographs.
Materials and methods
Fifty-three consecutive patients undergoing posterior lumbar interbody fusion (PLIF) for chronic low back pain were entered into the study. All patients who entered the study were given a detailed explanation of the study, the follow-up protocol and the radiological investigations, and their consent was obtained. There were 26 men and 27 women, between the ages of 30 and 79 years, with a mean age of 55 years. Two patients underwent three-level interbody fusion, 24 patients underwent two-level interbody fusion and 27 patients underwent single-level fusion. Three revision cases, one undergoing a three-level fusion and two undergoing a two-level fusion, for technical reasons had one of their levels fused through an anterior approach using a tricortical graft in two of the cases and a Ray cage in the third. There were 9 L3/4 levels fused, 39 L4/5, and 30 L5/S1, resulting in a total of 78 interbody levels fused. Since each level had two interbody cages, 156 cages were available for this study.
Surgical technique
All patients were operated in a prone position through a midline skin incision.
The initial stage involved insertion of posterior transpedicular instrumentation (Diapason, Stryker, UK) through a Wiltse paraspinal muscle splitting approach [12]. The transpedicular screws were inserted under lateral fluoroscopic guidance. The instrumentation was subsequently locked in distraction.
The next stage involved approaching the spinal canal through the midline. After completion of the decompression, which included doing facetectomies, each disc space to be fused was thoroughly prepared with the instruments on the set. Following this, two appropriately sized zero degrees titanium cages (Ogival, Stryker, UK), packed with local autogenous graft from the posterior elements, were inserted in each interbody space. The initially applied distraction was not relaxed at the time of insertion of the cages. The cages were inserted under lateral fluoroscopy so as to lie anteriorly in the interbody space. No bone graft was placed outside the cages in the interbody space. Also, posterolateral bone grafting was not performed. There were no instances where it was necessary to use bone graft from the iliac crest.
Post-operatively all patients were mobilized as their pain tolerance allowed in a canvas corset, which was retained for 3 months. All patients were reviewed prospectively, both clinically and radiologically, following a standardized protocol.
As part of the protocol, all the patients had a CT study and plain radiographs at 6 months post surgery. All the plain radiographs and CT scans were performed in a standardized fashion. CT studies consisted of 1- to 3-mm contiguous slices from the disc space above the cranial-most pedicle screws to the tips of the caudal-most pedicle screws. Coronal and sagittal multiplanar reconstruction (MPR) was routinely obtained through the cages.
CT scans and plain radiographs were reviewed blind and independently by a consultant radiologist (AS - observer 1) and an experienced spine research fellow (SM - observer 2). The results were recorded on a standardised form, designed to obtain a thorough evaluation of the radiological investigations and were evaluated by another research fellow (RRS). The following variables were assessed:
- Bridging bony trabeculation through the cages.
- CT scan: This was defined as the presence of bridging bony trabeculation through the cage from endplate to endplate without evidence of lucency along either margin as suggested by Cunningham et al. [8] and visualized on the sagittal and coronal MPR scans. This was also very well demonstrated by Kuslich et al. [18] in one of their patients who had a CT scan.
- Plain radiographs: This was defined as the continued presence of visible bone within the cage as suggested by Ray [27].
- Bridging bony trabeculation outside the cages: The space surrounding the cages was divided into lateral, posterior anterior and medial zones (Fig. 1).
- CT scan: This was defined as presence of bridging bony trabeculation on sagittal and coronal (MPR) scans in any of the zones mentioned above.
Fig. 1.
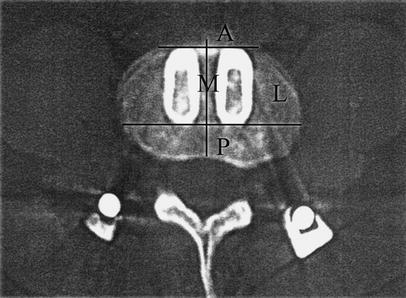
The space surrounding the cages was divided as shown into four zones: A anterior, L lateral, P posterior, M medial
-Plain radiographs: This was defined as presence of bridging bone in any of the zones mentioned above (essentially looking for a sentinel sign in any zone, in accordance with McAfee [25]), as seen on either the anteroposterior, lateral or the Ferguson view.
- 3.
Statistical methods
Inter-observer agreement was evaluated by calculating the Kappa co-efficient. The strength of agreement was based on the classification of Landis and Koch [9].
Chi-squared analysis determined the association between categorical variables, with the level of significance set at P<0.05.
Results
A total of 156 titanium interbody cages were available for review. The presence of titanium cages did not produce a significant deleterious effect on the quality of the CT scan.
Bridging bony trabeculation through the cages
On CT scan, both the observers noted presence of bridging bony trabeculation at L5/S1 in 56/60 cages (93%). At L4/5, observers 1 and 2 noted its presence in 74/78 cages (95%) and 76/78 cages (97%) respectively (Fig. 2), while at L3/4 both observers noted that all 18 cages (100%) had bridging bony trabeculation. Overall, therefore, observers 1 and 2 noted the presence of bridging trabeculation in 148/156 cages (95%) and 150/156 cages (96%) respectively, giving a Kappa value of 0.85 (almost perfect).
Fig. 2.
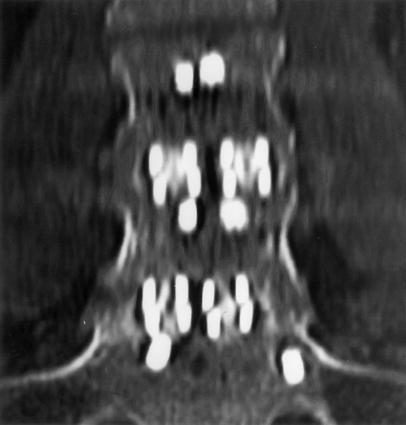
Computed tomographic (CT) coronal multiplanar reconstruction (MPR) of L4–S1 fusion. There was complete agreement between both observers on the presence of bridging bony trabeculation through all four interbody cages
On plain radiographs, both observers were able to identify bridging bone within the cages at L5/S1 in only 2/60 (3%). At L4/5, observers 1 and 2 were able to identify this in only 2/78 cages (3%) and 3/78 cages (4%) respectively, while at L3/4 observers 1 and 2 were able to identify it in 2/18 cages (11%) and 1/18 cages (6%) respectively. Overall, both were able to identify bridging trabeculation in only 6/156 cages (4%), giving a Kappa value of 0.74 (substantial).
Bridging bony trabeculation outside the cages
On CT scan, observer 1 identified trabeculating bone surrounding the cages in 138/156 cages (88.5%), while observer 2 observed this to be the case in 143/156 cages (91.7%), giving a Kappa value of 0.82 (almost perfect). When stratified by levels, observer 1 identified trabeculating bone surrounding 47/60 cages (78%) at L5/S1, 73/78 (94%) at L4/5, and 18/18 (100%) at L3/4, with the corresponding results for observer 2 being 50/60 (83%), 75/78 (96%) and 18/18 (100%). Therefore both observers noted increasing prevalence of bridging bony trabeculation from L5/S1 to L3/4. This was statistically significant for both observers, with the P-value being 0.006 for observer 1 and 0.01 for observer 2.
On plain radiographs, observer 1 identified surrounding trabeculating bone in 11/156 cages (7%), while observer 2 observed this to be present in 12/156 (8%), giving a Kappa value of 0.86 (almost perfect). When stratified by levels, observer 1 identified trabeculating bone surrounding 3/60 cages (5%) at L5/S1, 2/78 (3%) at L4/5, and 6/18 (33%) at L3/4, with the corresponding results for observer 2 being 2/60 (3%), 2/78 (3%) and 8/18 (44%). These observations mirrored the CT scan findings of increasing prevalence of bridging bony trabeculation from L5/S1 to L3/4. This was again statistically significant, with P<0.001 for both observers.
The results were evaluated further to determine any differences between single- and two-level fusions. Three-level fusions were not compared, as there were only two cases. On the CT scan, both observers noted that there was an absence of surrounding trabeculating bone around either one or both cages in 2/27 cases (7%) of single-level fusion, while the corresponding incidence for two-level fusion was 6/24 cases (25%) and 4/24 (17%) for observers 1 and 2 respectively. This was not statistically significant, with the P-value being 0.08 for observer 1 and 0.31 for observer 2. On plain radiographs, both observers noted the trabeculation to be present in 3/27 cases (11%) of single-level fusion, with the corresponding incidence for the two-level fusion being 2/24 cases (8%) and 3/24 (13%) for observers 1 and 2 respectively. This again was not statistically significant, with the P-value being 0.74 for observer 1 and 0.88 for observer 2.
Identification of trabeculating bone in relation to individual zones is detailed in Table 1. Overall, both observers noted that the lateral zone was most likely to show bony trabeculation at all levels, and this difference between the zones was statistically significant, with P<0.001 for both observers (Fig. 3).
Table 1.
Bridging bony trabeculation outside the cage zone and level distribution (Obs observer)
| Level | Lateral | Anterior | Posterior | Medial | ||||
|---|---|---|---|---|---|---|---|---|
| Obs 1 | Obs 2 | Obs 1 | Obs 2 | Obs 1 | Obs 2 | Obs 1 | Obs 2 | |
| L3/4 (n=18) | 16 (89%) | 16 (89%) | 13 (72%) | 14 (78%) | 14 (78%) | 14 (78%) | 6 (33%) | 7 (33%) |
| L4/5 (n=74) | 58 (74%) | 67 (86%) | 48 (62%) | 65 (78%) | 45 (58%) | 67 (86%) | 42 (54%) | 46 (59%) |
| L5/S1 (n=60) | 38 (63%) | 48 (80%) | 20 (33%) | 24 (40%) | 21 (35%) | 31 (52%) | 8 (13%) | 18 (30%) |
| Total (n=156) | 112 (72%) | 131 (84%) | 81 (52%) | 103 (66%) | 80 (51%) | 112 (72%) | 56 (36%) | 71 (46%) |
Fig. 3.
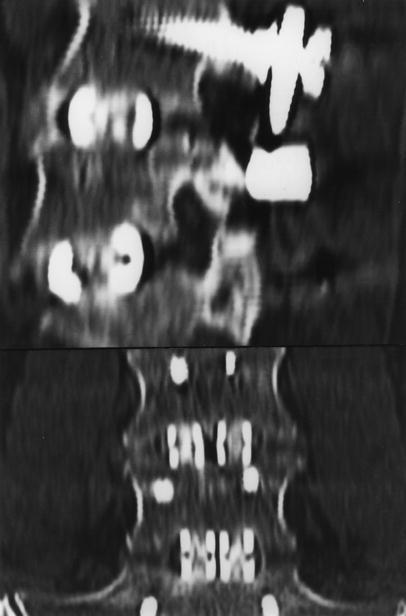
Sagittal and coronal CT MPR of L4–S1 fusion (different case from Fig. 2) exhibiting bridging bony trabeculation around the cages. Note especially the presence of bony trabeculation in the anterior zone at L4/5 and the medial corticalisation of lateral zone bone formation of the left L5/S1 cage
Cage endplate interface
On the CT scan, observer 1 noted the presence of a radiolucent cage endplate interface around 10/156 cages (6%), while observer 2 identified this around 6/156 (4%), giving a Kappa value of 0.74 (substantial). When stratified by levels, observer 1 identified the presence of a radiolucent interface around 6/60 cages (10%) at L5/S1, 4/78 (5%) at L4/5, and 0/18 at L3/4, with the corresponding results for observer 2 being 4/60 (7%), 2/78 (3%), and 0/18 respectively. Overall, there was complete agreement between the two observers that six cages—four at L5/S1 and two at L4/5—had a radiolucent interface around them, and were considered to be not fused (Fig. 4).
Fig. 4.
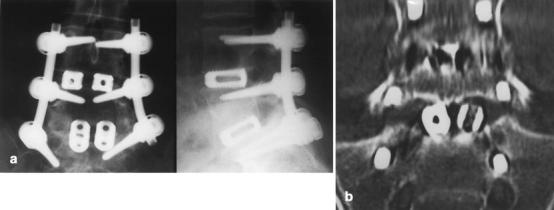
A Anteroposterior and lateral radiographs of L4–S1 fusion. There was complete agreement between the two observers that there was no evidence of loosening in relation to any of the cages. Also note the presence of bone within the cages at L4/5. B. Sagittal CT MPR of the same case. Both observers agreed on the presence of a radiolucent interface between the left L5/S1 cage and the L5 endplate, indicating that it was not fused
On the plain radiographs, observer 1 noted the presence of a radiolucent cage endplate around 4/156 cages (3%), while observer 2 identified this around 2/156 (1%), giving a Kappa value of 0.66 (substantial). When stratified by levels, at L5/S1, observers 1 and 2 noted the presence of a radiolucent interface around 4/60 cages (7%) and 2/60 (3%) respectively, while at L4/5 and L3/4 neither observer was able to detect a radiolucent cage endplate interface. Overall, there was complete agreement between the two observers that two cages at L5/S1 had a radiolucent interface and were therefore considered to be not fused.
Discussion
The availability of lumbar interbody cages has fuelled renewed interest in interbody fusion, with over 80,000 lumbar interbody cages being inserted world-wide in the 5-year period prior to 1999 [23]. Despite this, there is no consensus regarding the best non-invasive method for evaluation of interbody fusion, especially where cages have been used. This was more than clearly evident from a recent symposium [24], in which there was considerable disagreement among eight different authors regarding the best method as well as the criteria of each method to assess fusion.
In 1948, Bosworth [3] stated in relation to posterior spinal fusion that: "the only way to be sure about the status of the fusion is to explore it". It would be reasonable to assume that the same held true for interbody fusion at that time. In the intervening 50 years, there is still no agreement on the best way of determining fusion other than surgical exploration, which continues to remain the "gold standard" [5, 6].
Trying to follow Bosworth's dictum would entail routine exploration with removal of the posterior instrumentation, if used, to assess the fusion. There are obvious inherent practical problems with this approach. It is probably neither viable nor justifiable to re-operate on each patient undergoing lumbar fusion, irrespective of the approach used, especially where post-operatively the patient has minimal or no symptoms. Additionally, unlike a posterolateral fusion, direct visualization is not possible in an interbody fusion from a posterior approach. Also, there has been no study assessing the amount of distraction necessary to demonstrate movement between the vertebral bodies. However, it would be reasonable to assume, as has been observed by Fraser [24], that with the patient lying prone, considerable distraction force may be required to demonstrate any movement, and a pseudarthrosis may be missed. It therefore becomes imperative to have a reliable non-invasive method of assessing fusion.
Bridging bony trabeculation through the cages
Blumenthal and Gill [1], on routine removal of metalwork in 49 patients following combined interbody and posterolateral fusion at 9 months, found that plain radiographs were able to predict the presence or absence of arthrodesis in roughly two-thirds of the cases. They also observed that plain radiographs underestimated fusion in one in five cases. Brantigan et al. [5], using plain radiographs, reported 100% (91/91) radiographic arthrodesis in their subgroup of degenerative disc disease patients treated with their radiolucent cage at 24 months. Closer evaluation of their results, however, shows that at 6 months, only 55% (50/91) were noted to be showing arthrodesis.
Ray [27] described the findings of living bone in biopsies from four cages performed 6–12 months post surgery.
Boden et al. [2], using threaded hollow cylindrical titanium interbody cages to assess rhBMP-2 in rhesus monkeys, found that plain radiographs were of limited value, as the titanium cage obscured reliable assessment of bone formation. They did, however, find that sagittally reformatted CT scans were considerably better. In the same experiment they showed histologically that by 24 weeks there was continuous trabecular bone growth through the cage, which correlated with the sagittal CT findings.
Cunningham et al. [8], in their study of rhOP-1 using BAK cages in sheep thoracic spine, found a 100% correlation between CT and histological microradiographs in the BAK-autograft group. They were able to demonstrate at 4 months in all specimens classified as fused, definitive evidence of contiguous well-organized bony trabeculation through the BAK cages spanning the fusion site from the adjacent vertebral bodies. They also noted that accurate assessment of a successful arthrodesis using plain film was uncertain due to its inability to visualize the bone within the cage.
Eck et al. [10], in their evaluation of fusion following use of titanium mesh cages, also found it difficult to evaluate intracage fusion mass using plain radiographs.
In our study, both observers were able to identify the presence of bridging bony trabeculation through the cages on plain radiographs at 6 months in only 4%, as compared to the 95% on the CT scan. The findings of this study therefore appear to suggest that crossing bony trabeculation through an interbody cage using autologous bone graft can be demonstrated at 6 months on thin-slice sagittal and coronal reconstruction CT images. Even though Boden [24] suggests that CT scan at this stage does not allow us to differentiate between live and avascular bone, we would tend to agree with Kuslich [18, 24], Ray [24, 27] and Zdeblick [24], who feel that the presence of bridging bony trabeculation through the cages as seen on thin-slice CT scan is a reasonable criterion to determine fusion. The CT scan findings would also be consistent with those of Ray [27], who found evidence of early bone bridging in the biopsies performed at 6–12 months post surgery in four of his cages.
The very low incidence of bridging bony trabeculation on plain radiographs, as noted by both observers, may be secondary to a number of factors. It may be due to difficulty in assessing this in the presence of a metallic cage, as noted by Eck et al. [10]. It may also be that plain radiographs underestimate fusion at 6–9 months, as noted by Blumenthal and Gill [1], and evident from the results of Brantigan et al. [5], as noted above.
It would appear, therefore, that CT may have significant potential clinical value in earlier prediction of satisfactory interbody fusion following the use of interbody cages. However, a long-term follow-up of this cohort will be necessary to see whether the current CT predictions are proved correct and for the determination of the false-positive rate.
Bridging bony trabeculation outside the cages
McAfee et al. [25] felt that the most reliable radiographic indicator of fusion was the presence of bridging bone anterior to the fusion cage, the so-called "sentinel" sign. To help achieve this goal, he advocated packing bone graft anterior to the cages (the cages being inserted through an anterior approach). Fraser [24] also felt that the presence of continuous trabeculated bone external to the cage was necessary for pronouncement of a successful arthrodesis.
McAfee et al. [25], in a series of endoscopic interbody fusions using BAK cages, noted trabecular bony bridging across adjacent vertebrae laterally by 6 months in 15/15 patients. However, in a subsequent symposium [24], McAfee does mention that in over 200 cases using BAK cages with bone again deliberately placed exterior to the cage, the sentinel sign was confirmed in 80% of the cases. He did not provide any more information in relation to the levels or timing with this statement.
One interesting outcome of the evaluation of CT scans in our study was the detection of crossing bony trabeculation outside the cages despite the fact that no bone grafts had been placed in the interbody space external to the cage. Ray [27] mentions that four out of ten patients in his initial group had visible annular ossification surrounding the cages, when followed for more than 2 years post-operatively. Brantigan et al. [5] describe bone extending to fill the disc space at 2 years in one of the cases illustrated in their article, although it is not clear whether this was a regular occurrence, nor whether bone graft had been placed around the cages.
Eck et al. [10], in their review of titanium mesh cages, noted that CT with sagittal reconstruction would be a useful tool for assessing fusion mass around the cages.
In our study, both observers identified apparent bridging bony trabeculation around 90% of the cages on CT scan, compared to around 7% on plain radiographs. Further analysis of the CT scan findings indicated this was most likely to be present at L3/4, and least likely at L5/S1. This was statistically significant for both observers (P=0.01 for observer 1 and P=0.006 for observer 2). Interestingly, even though only 7% of cages were noted to show bridging bony trabeculation around them, further evaluation indicated that it mirrored the CT scan pattern of increasing prevalence of bridging bony trabeculation from L5/S1 to L3/4. This was again statistically significant (P<0.001 for both observers).
When stratified by zones, the bony trabeculation was most commonly present in the lateral, posterior and anterior zones. The latter was even more surprising since the cages were placed anteriorly in the disc spaces. Overall, the bone was most likely to be visible in the lateral zone as compared to the other zones, which was statistically significant (P<0.001 for both observers).
This is the first study that appears to show the presence of consistent bone formation outside the cages in the lumbar spine, in spite of not placing any bone graft external to the cage in the interbody space. This finding is of great importance, especially since the presence of continuous trabeculated bone external to the cage is thought to be necessary for a successful interbody fusion by certain authors [24].
Cage endplate interface
Stauffer and Coventry [29], in their review of the Mayo clinic series of anterior lumbar interbody fusion using autograft, found that in most patients in whom pseudarthrosis developed, it became obvious within 6–9 months following surgery. They used plain radiographs, including flexion-extension views, as well as tomograms to assess fusion. Cleveland et al. [7] used biplane bending radiographs to assess posterior spinal fusion in 594 patients. Interestingly, they also noted that if a pseudarthrosis was to develop, it usually became apparent within 8 months following the surgery. Kozak and O'Brien [17], in their study of 69 patients undergoing simultaneous combined anterior and posterior fusion using autograft, also noted that interbody fusion was unequivocal on plain radiographs in most cases by 6–9 months. Yashiro et al. [31], in their study of instrumented PLIF using autograft and the Steffee VSP system, used the criteria of increasing bone density and obscuring of the boundary between the grafted bone and vertebral body with absence of an apparent radiolucent zone on plain radiographs to assess fusion. They found bony union in 28/30 (93%) of their cases within a mean of 6.3 months. These studies, we feel, support our decision to assess the fusion at 6 months following surgery.
In our study, there was unanimity between the two observers regarding the presence of a radiolucent interface around six cages on CT scan, as compared to two cages on plain radiographs. The use of posterior transpedicular instrumentation may limit the value of flexion-extension views on plain radiographs. Our findings therefore suggest that CT may be the investigation of choice for early detection of a radiolucent interface around interbody cages.
This study has compared plain radiographs and high-quality thin-section CT scan with coronal and sagittal reconstruction at 6 months following spinal fusion. For the practical clinical reasons described, the comparative data for the currently adopted "gold standard" of surgical exploration is not available. However, the results presented suggest that CT provides a more sensitive assessment of interbody fusion at 6 months than plain radiographs, with a more robust inter-observer agreement.
Future review of this cohort of patients will allow correlation of 2- and 5-year plain radiographic findings with the CT appearances at 6 months, which may quantify the specificity of this technique.
Conclusions
At 6 months following transpedicular instrumentation and surgical interbody fusion with titanium cages packed with local bone from decompression:
CT shows images suggesting bridging bony trabeculation through 95% of the cages.
CT findings also suggest new bone formation in the interbody space in the absence of formal grafting outside 90% of the cages.
Plain radiographs show bridging bone in 4% of cages and bone surrounding 7% of cages.
CT is more likely to detect early development of a radiolucent interface around an interbody cage (4%) as compared to plain radiographs (1%).
Potential for the future:
CT may be used to predict pseudarthrosis earlier then plain radiographs by identifying a pathological interface between the cage and the vertebral endplate.
CT may predict fusion earlier than plain radiographs, leading to shorter post-operative follow-up.
Acknowledgement
The authors acknowledge the statistical advice given by Dr. Richard Morris, Department of Primary Care and Population Sciences, Royal Free & University College Medical School, London, UK.
Footnotes
The work for this study was carried out at the Spinal Surgical Unit of the Royal National Orthopaedic Hospital. No financial support of any kind has been received from any party for the purpose of this study
References
- 1.Blumenthal Spine. 1993;18:1186. doi: 10.1097/00007632-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Boden J Spinal Disord. 1998;11:95. [PubMed] [Google Scholar]
- 3.Bosworth AAOS Instructional Course Lecture. 1948;5:295. [Google Scholar]
- 4.Brantigan Spine. 1993;18:2106. doi: 10.1097/00007632-199310001-00030. [DOI] [PubMed] [Google Scholar]
- 5.Brantigan Spine. 2000;25:1437. doi: 10.1097/00007632-200006010-00017. [DOI] [PubMed] [Google Scholar]
- 6.Brodsky AE, Kovalsky ES, Khalil MA (1991) Correlation of radiologic assessment of lumbar spine fusions with surgical exploration. Spine [Suppl] 16:261–265 [DOI] [PubMed]
- 7.Cleveland J Bone Joint Surg Am. 1948;30:302. [PubMed] [Google Scholar]
- 8.CunninghamSpine 19992450910101812 [Google Scholar]
- 9.Dunn G, Everitt B (1995) Clinical biostatistics. An introduction to evidence based medicine. Edward Arnold, London
- 10.Eck Spine. 2000;25:2407. doi: 10.1097/00007632-200009150-00023. [DOI] [PubMed] [Google Scholar]
- 11.Fagan AB, Cain CMJ, Hall DJ, Fraser RD (1999) Carbon fiber cages for anterior lumbar interbody fusion: a prospective study. In: Szpalski M, Gunzburg R, Pope MH (eds) Lumbar segmental instability.Lippincott Williams Wilkins, Philadelphia, pp 203–208
- 12.Fraser Bull Hosp Joint Dis. 1996;55:158. [Google Scholar]
- 13.Greenough Eur Spine J. 1994;3:225. doi: 10.1007/BF02221598. [DOI] [PubMed] [Google Scholar]
- 14.Hacker Spine. 1997;22:660. doi: 10.1097/00007632-199703150-00017. [DOI] [PubMed] [Google Scholar]
- 15.Herzog Spine. 1996;21:1114. doi: 10.1097/00007632-199605010-00027. [DOI] [PubMed] [Google Scholar]
- 16.Holte Eur Spine J. 1994;3:32. doi: 10.1007/BF02428314. [DOI] [PubMed] [Google Scholar]
- 17.Kozak Spine. 1990;15:322. doi: 10.1097/00007632-199004000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Kuslich Spine. 1998;23:1267. doi: 10.1097/00007632-199806010-00019. [DOI] [PubMed] [Google Scholar]
- 19.Laasonen Spine. 1989;14:210. doi: 10.1097/00007632-198902000-00011. [DOI] [PubMed] [Google Scholar]
- 20.LangSpine 198813693381143 [Google Scholar]
- 21.Larsen J Spinal Disord. 1996;2:117. [PubMed] [Google Scholar]
- 22.Leufven C, Nordwall A (1999) Management of chronic disabling low back pain with 360 degrees fusion. Results from pain provocation test and concurrent posterior lumbar interbody fusion, posterolateral fusion, and pedicle screw instrumentation in patients with chronic disabling low back pain. Spine. 24:2042–2045 [DOI] [PubMed]
- 23.McAfee J Bone Joint Surg Am. 1999;81:859. doi: 10.2106/00004623-199906000-00014. [DOI] [PubMed] [Google Scholar]
- 24.McAfee Spine. 2001;26:320. doi: 10.1097/00007632-200102010-00020. [DOI] [PubMed] [Google Scholar]
- 25.McAfee Spine. 1998;23:1476. doi: 10.1097/00007632-199807010-00009. [DOI] [PubMed] [Google Scholar]
- 26.Pearcy J Bone Joint Surg Br. 1982;64:228. doi: 10.1302/0301-620X.64B2.7040410. [DOI] [PubMed] [Google Scholar]
- 27.Ray Spine. 1997;22:667. doi: 10.1097/00007632-199703150-00019. [DOI] [PubMed] [Google Scholar]
- 28.Rothman Radiology. 1984;150:185. doi: 10.1148/radiology.150.1.6227935. [DOI] [PubMed] [Google Scholar]
- 29.Stauffer J Bone Joint Surg Am. 1972;54:756. [PubMed] [Google Scholar]
- 30.Weiner Spine. 1998;23:634. doi: 10.1097/00007632-199803010-00020. [DOI] [PubMed] [Google Scholar]
- 31.Yashiro Spine. 1991;16:1329. doi: 10.1097/00007632-199111000-00014. [DOI] [PubMed] [Google Scholar]


