Abstract
SINE (short interspersed element) insertion analysis elucidates contentious aspects in the phylogeny of toothed whales and dolphins (Odontoceti), especially river dolphins. Here, we characterize 25 informative SINEs inserted into unique genomic loci during evolution of odontocetes to construct a cladogram, and determine a total of 2.8 kb per taxon of the flanking sequences of these SINE loci to estimate divergence times among lineages. We demonstrate that: (i) Odontocetes are monophyletic; (ii) Ganges River dolphins, beaked whales, and ocean dolphins diverged (in this order) after sperm whales; (iii) three other river dolphin taxa, namely the Amazon, La Plata, and Yangtze river dolphins, form a monophyletic group with Yangtze River dolphins being the most basal; and (iv) the rapid radiation of extant cetacean lineages occurred some 28–33 million years B.P., in strong accord with the fossil record. The combination of SINE and flanking sequence analysis suggests a topology and set of divergence times for odontocete relationships, offering alternative explanations for several long-standing problems in cetacean evolution.
Keywords: SINE, evolution, divergence times
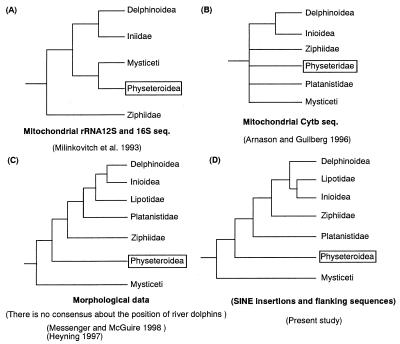
Extant whales, dolphins, and porpoises (order Cetacea; refs. 1 and 2) are usually grouped into two suborders, Odontoceti (echolocating toothed whales) and Mysticeti (filter-feeding baleen whales), both of which were thought to be monophyletic on the basis of morphological, physiological, and behavioral characteristics (3–5). The extant-toothed whales have been divided into 4 broad groups consisting of 10 families: sperm whales (Physeteroidea–families Physeteridae and Kogiidae), beaked whales (family Ziphiidae), river dolphins (4 families, below), and ocean dolphins or delphinoids (Delphinoidea–families Monodontidae, Delphinidae, and Phocoenidae) (4). Among these four broad groups, the physeteroids are usually basal (e.g., refs. 6 and 7), and the delphinoids are the most recently diverged. River dolphins often are placed as sisters to delphinoids, and beaked whales have either been allied with river dolphins and delphinoids or placed together in a clade with sperm whales. Since Milinkovitch et al. (8–10) proposed the paraphyly of the Odontoceti, suggesting sperm whales are closer to the morphologically highly divergent baleen whales than to other Odontoceti (Fig. 1A), the phylogenetic position of sperm whales has been debated widely (8–14). Another contentious issue is the relationships among river dolphins, which have long been placed in up to four monotypic subfamilies or families (4). These species are the Ganges River and Indus River dolphins (Platanistidae, Platanista gangetica–1 or 2 species), Amazon River dolphins (Iniidae, Inia geoffrensis), La Plata dolphins (Pontoporiidae, Pontoporia blainvillei) and the Yangtze River dolphins (Lipotidae, Lipotes vexillifer). Because river dolphins are similar in external appearance and/or habits, or for nomenclatural convenience, cetologists historically placed river dolphins in a single higher group, the Platanistoidea (15). However, the appropriateness of this grouping has been doubted long by both morphologists and molecular phylogeneticists (refs. 4, 6, 12, 13, 16, 17, 18, and 19; Fig. 1), and the debate is ongoing. Uncertainty about the phylogeny of river dolphins reflects not only high skeletal disparity among living species, but also a fragmentary fossil record that reveals little about origins. Fig. 1 summarizes several different hypotheses of odontocete relationships.
Figure 1.
Four proposed phylogenetic trees among the major lineages of cetaceans. (A) The Milinkovitch tree (8). (B) The cytochrome b tree deduced by Arnason and Gullberg (12). (C) A majority tree by morphological data. (D) The tree of the present study. Phylogenetic position of Physeteroidea (sperm whales) was boxed.
To elucidate odontocete phylogeny, we adopted the SINE (short interspersed element) insertion method (20–25). As a consequence of the replicative mechanism of retroposons, the integration of a SINE at a new locus is an irreversible event, and the sites of such integration are distributed randomly throughout the genome. The probabilities that a SINE will be removed without detection or inserted into the same independent locus in unrelated lineages are infinitesimally small, thus homoplasy and character conflicts are very unlikely (20, 21, 25) and problems of ingroup sampling (e.g., long branch attraction) are negligible. Because the polarity of SINE insertion is fixed (absence vs. presence), outgroup identification is straightforward and free from artifacts of taxon sampling (20, 25). Recently, the SINE method has clarified successfully previously contentious phylogenies of salmonid fishes (22), of African cichlid tribes (23), and of whales in relation to even-toed ungulates (24, 25). The method has become an attractive and powerful tool to complement the use of DNA sequence comparisons in phylogeny.
Materials and Methods
Fourteen cetacean species (3 mysticetes and 11 odontocetes) were examined in this study, with the hippopotamus as an outgroup. DNA clones were screened from a genomic library for the presence of the given SINE unit by using either the CHR-1 or CHR-2 SINE sequence (24, 25) as a probe. Positively hybridizing clones were identified and sequenced. Primers nested in the flanking sequence of the particular SINE unit were designed. Sequence information for primers is available on request. PCR and other experimental procedures were performed by standard techniques (26–28). PCR amplification was conducted followed by electrophoretic visualization of size-dimorphic bands (fragments possessing or lacking target SINE inserts). Final confirmation of the presence or the absence of the SINE in the locus was obtained by sequencing. The nucleotide sequence data have been deposited in GenBank (AB054370–AB054523).
For phylogenetic analysis, the SINE insertion data were organized into a transformation series, where the absence of a retroposon at a particular locus was coded as 0 and the presence of a retroposon at that same locus was coded as 1 (Fig. 2). The parsimony program PAUP* (29) was used to reconstruct phylogenetic relationships among taxa (Fig. 3). By using concatenated sequences of the 12 flanking loci (2792 nucleotides in total excluding insertion/deletion sites), the branching orders among 7 major taxa were estimated by the baseml program (30) with the HKY + Γ model (31, 32). The Bayesian method (33) was used for estimation of branching times.
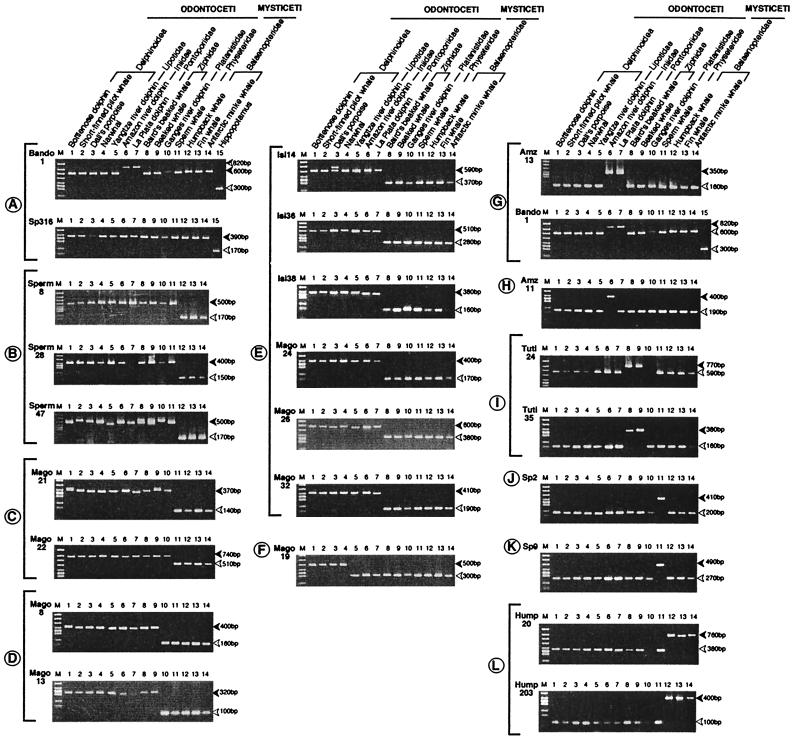
Figure 2.
Characterization of 25 SINE loci by PCR. In each locus, filled and blank arrowheads show bands with the presence and absence of a SINE unit, respectively. In the Bando1 locus, an additional insertion is indicated. Each clade is shown alphabetically from A to L. Of the 25 loci described herein, Sperms (nos. 8, 28, and 47), Isis (nos. 14, 36, and 38), Magos (nos. 8, 13, 19, 21, 22, 24, 26, and 32), Tutis (24 and 35), Sps (nos. 2, 9, and 316), Amzs (nos. 11 and 13), Humps (nos. 20 and 203), and Bando1 were newly isolated by cloning and sequencing from genomic libraries of Dallís porpoise, short-finned pilot whale, sperm whale, Amazon River dolphin, humpback whale, and bottlenose dolphin, respectively.
Figure 3.
Phylogenetic relationships among the major lineages of cetaceans. Newly isolated and characterized loci are boxed. [Reproduced with permission from ref. 57 (Copyright 1998, Simon & Schuster).]
Results
Fig. 2 shows PCR patterns of 25 informative SINE loci, using a filled arrowhead to indicate a SINE-presence locus. Because the PCR pattern is unambiguous, the species with a SINE in each locus can be grouped as a clade, leading to the generation of a unique cladogram shown in Fig. 3 [corroborated by maximum parsimony analysis using PAUP* (29)]. Fig. 4 shows compilation of parts of sequences of the representative 11 SINE loci.
Figure 4.
Compilation of parts of sequences of the 11 representative SINE loci. The name of the SINE family as well as its subfamily (in parenthesis) is indicated in a bold box. CD (Cetacean-specific deletions) and CDO (CD specifically present in Odontoceti) were characterized recently as subfamilies of the CHR-2 SINE family (M.N. and N.O., unpublished data). Direct repeats are shadowed. Identical nucleotides are shown by dots, and deletions are shown by bars.
Two newly isolated SINE loci (Figs. 2 and 3, clade A), together with three previously characterized loci (Pm72, Pm52, and M11), clearly indicate the monophyly of Cetacea. The monophyly of odontocetes (including sperm whales) is recorded by 3 independent SINE insertion events (Figs. 2 and 3, clade B). Furthermore, SINE loci also indicate the branching order of the primary odontocete lineages as sperm whales (clade B), Ganges River dolphins (clade C), beaked whales (clade D), and finally the marine and remaining freshwater dolphins (clade E). Locus patterns unambiguously demonstrate the polyphyly of river dolphins [Platanistoidea sensu (15)]. One SINE locus (Figs. 2 and 3, clade F) supports the monophyly of ocean dolphins (Delphinoidea), and two loci (Figs. 2 and 3, clade G) show a sister relationship between the two South American dolphins, Inia and Pontoporia. (The relationship of Lipotes is resolved by using flanking sequences described below.) Seven SINE insertions indicate clades from H to L (Figs. 2 and 3). For example, the Amz11 locus indicates a species-specific insertion for the Amazon river dolphins. The Sp2 locus indicates a species-specific insertion for the Pygmy sperm whales, whereas the Sp9 locus indicates an insertion in a common ancestor of the Sperm and the Pygmy sperm whales.
Because SINEs are inserted into unique orthologous loci, their flanking sequences can provide phylogenetic information (34). We analyzed SINE flanking sequences to resolve the relationships between the South American river dolphins (Inia and Pontoporia) and the Yangtze River dolphins (Lipotes). Contrary to recent molecular phylogenetic analyses (18, 19), we found strong support for monophyly of the Yangtze and South American river dolphins (99% bootstrap value). The SINE flanking-sequence analysis finds moderate support for odontocete monophyly with a 72% bootstrap value and firmly rejects the baleen/sperm whale grouping, which has only a 4% bootstrap value.
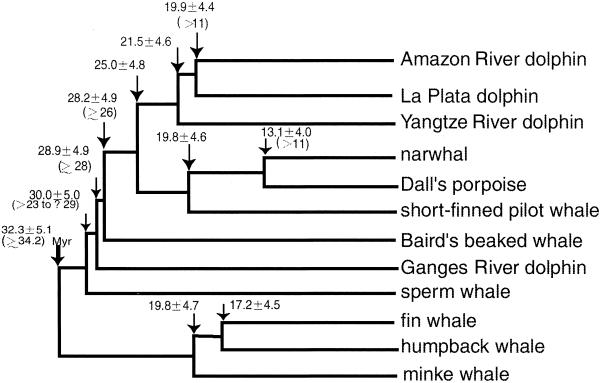
To predict the timing of phylogenetic events, the relaxed clock of cytochrome b amino acid sequences (data from ref. 12) was calibrated first with a 55-million-year (Myr) date for the separation of Cetacea from the hippopotamus (35). Using this calibration, the baleen/toothed whale separation was estimated at 32.3 +/−5.1 Myr B.P. (+/−: 1SE), and then this date was used in calibrating the relaxed clock of SINE flanking sequences (with the HKY model). The major clades of odontocetes have estimated divergence dates from about 25–30 Myr B.P. (Fig. 5). Overall, these estimates suggest a very rapid early radiation among the major groups of odontocetes and mysticetes.
Figure 5.
Estimates of branching dates in the cetacean evolution by using the relaxed molecular clock of SINE flanking sequences. Branching times estimated from fossil data are indicated in parenthesis.
Discussion
The SINE method is a new and powerful tool for exploring phylogeny. Here, three independent SINE loci (Figs. 2 and 3 clade B) plus the analysis of SINE flanking sequences clearly indicate odontocete monophyly. There is no support for alternative molecular hypotheses of odontocete paraphyly (8–11, 36, 37). The SINE results clearly separate Platanista from the other river dolphins, thus supporting morphologically based concepts of Platanistoidea and Delphinida (Delphinoidea + Inioidea + Lipotes; refs. 6, 16, 17, 38, and 39), but suggest a surprising phylogenetic position for Platanista. Until now, it seemed that Platanista branched after the divergence of sperm and beaked whales (refs. 6, 7, and 13 but see ref. 40; Fig. 1C). Recent molecular analyses have placed Platanista and beaked whales in a clade between sperm whales and more crown-ward odontocetes (18) or, notably, have placed Platanista between sperm and beaked whales (19). Here, the 10 SINE loci in clades C, D, and E confirm that Platanista branched after the divergence of sperm whales but before the divergence of beaked whales.
The proposed near-basal position for Platanista within the Odontoceti has significant implications. Sperm and beaked whales are neither sister-taxa (6, 40) nor adjacent clades (7), and morphological similarities between these lineages could be plesiomorphies or convergences associated with deep-diving behavior and/or suction-feeding. These similarities involve skull complexes traditionally given significant phylogenetic value, including the pterygoid sinuses and ear bones (6, 41, 42).
We know of no anatomical features that preclude the proposed position for Platanista within the Odontoceti. Presumed synapomorphies [cited previously (6, 7)] to support a more crown-ward position for Platanista are perhaps plesiomorphies, autapomorphies, or homoplasies. Different interpretations of structure arise because, anatomically, Platanista is one of the most peculiar mammals (43). The skull is highly disparate from other Cetacea, especially in having high pneumatized maxillary crests that arch over the face, probably acting as acoustic reflectors for echolocation sounds generated in the underlying soft nasofacial tissues. The pneumatic sinuses within the maxillary crests arise ventrally in the pterygoid sinus complex of the skull base (41). Sperm and beaked whales have simple large pterygoid sinuses with reduced bony walls, and complex multilobed sinuses occur elsewhere only in the Delphinida (Delphinoidea + Inioidea + Lipotes) (41). We conclude that complex multilobed sinuses have evolved at least twice in odontocete phylogeny. Further, unlike the Inioidea and Lipotes, the fossil record for the Platanista clade is long and extensive, including the Platanistidae and the extinct Squalodelphinidae, Dalpiazinidae, Waipatiidae, and Squalodontidae (5, 6, 16, 40). Fossils show that complex multilobed sinuses evolved in the Squalodelphinidae, and that sinus-bearing maxillary crests appeared in the Platanistidae.
Morphologists (6, 7) and molecular phylogeneticists (18, 19) disagree about the relationships of Inia, Pontoporia, Lipotes, and ocean dolphins (Delphinoidea), and almost every combination of taxa has been proposed. Heyning (7) clustered Inia, Pontoporia, and Lipotes together, whereas Barnes (44) proposed a sister relationship between Pontoporia and Lipotes, excluding Inia. Alternatively, others (6, 18, 19) have proposed a sister relationship among Inia, Pontoporia, and ocean dolphins (Delphinoidea), excluding Lipotes. Here, SINE flanking-sequence analysis strongly supports a clade for Inia, Pontoporia, and Lipotes to the exclusion of ocean dolphins (Delphinoidea). Establishing the monophyly of three such geographically disjunct lineages is a critical step toward understanding the evolutionary history of these enigmatic animals.
Our study identifies a rapid radiation of extant cetaceans at about 28–33 Myr B.P. (Fig. 6) in the Oligocene Epoch. This is a pioneering result for molecular studies in that it is strongly consistent with the fossil record of Odontoceti and Mysticeti. The oldest reported fossil mysticete is the archaic toothed Llanocetus denticrenatus, dated at about 34.2 Myr B.P. (45–47) and, presumably, the sister taxon Odontoceti had appeared by that time (5). There is no compelling fossil evidence of an older origin, 40–45 Myr, for Mysticeti + Odontoceti within the stem Cetacea (Archaeoceti) (cf. 18). Among archaeocetes, the putative sister taxa for Mysticeti + Odontoceti are the Late Eocene species of Saghacetus and Zygorhiza dated at 35–36 Myr B.P. (48)—barely older than Llanocetus. Further, the relatively dense Eocene record of Cetacea beyond 34 Myr B.P. (e.g., ref. 49) has produced no beaked whales, platanistoids, sperm whales, or mysticetes. Thus, the calculated divergence dates of Cassens et al. (18) seem markedly too old. Equally, an origin for baleen whales at 25 Myr B.P. (50) is 8–9 Myr younger than what has been predicted by SINEs and what is known from the fossil record.
The rapid radiation of extant cetaceans predicted by SINE methods is elucidated by geological processes. Fossil cetaceans are rare in the interval from 34 to 29–30 Myr B.P., probably because changing global sea levels (caused by a fluctuating Antarctic ice-cap) eroded bone-rich strata (5, 51). The global record, however, is excellent in the Late Oligocene interval (about 29–30 to 23 Myr B.P.). Late Oligocene fossils include early sperm whales, archaic Delphinoidea, many Platanistoidea (Squalodelphinidae, Dalpiazinidae, Waipatiidae, and Squalodontidae), and diverse Mysticeti (5). This record indicates a major explosive radiation of the Cetacea in Early Oligocene times (34–29 Myr B.P.), immediately after the archaeocete to mysticete–odontocete transition of 34–35 Myr B.P. This Early Oligocene radiation was concurrent with major shifts in global climate (e.g., refs. 52 and 53) and ocean productivity (54), linked to new marine circulation patterns resulting from the final breakup of Gondwanaland and the opening of the Southern Ocean. The cetacean radiation involved radical and divergent shifts in feeding strategies, with the evolution of filter-feeding in Mysticeti and echolocation-assisted predation in Odontoceti (51). The cetacean radiation is explained through a cascade of changing oceanic food chains, productivity, climate, circulation, and continental breakup (45, 51). A rapid taxonomic and ecological radiation of cetaceans, with many lineages appearing and diversifying over about 5 Myr, plausibly explains why the previous sequence analyses (8, 12) did not give clear estimates.
A key result of the SINE work is the unexpected phylogenetic position of Platanista, a dolphin that differs dramatically in biology from its neighboring clades of sperm and beaked whales. Platanista now includes only one or two living species (Ganges/Indus River dolphins; ref. 43), but its lineage, the Platanistoidea, was highly successful in the past 30 Myr, judging from many fossil species from marine strata around the world. Fossil platanistoids show a high family-level diversity (described above, and e.g., refs. 5, 6, and 16), indicating substantial ecological partitioning in this lineage. Fossils also reveal that the Platanistidae was marine from its beginnings with Zarhachis [about 16 Myr B.P. (55)]; at least until the appearance of Pomatodelphis in marine strata of Florida at 10–11 Myr B.P. (56). Pomatodelphis is the putative sister taxon to Platanista (38), but there is a roughly 10-Myr gap between records of Pomatodelphis in Atlantic marine rocks, and Platanista, with no fossil record, in the fresh waters of the Indian subcontinent. When platanistids invaded fresh waters is uncertain; but the decline in platanistoid taxa matches the explosive radiation of delphinoids (especially Delphinidae) later in the Miocene (about 11–12 Myr B.P.), and perhaps platanistoids were displaced by delphinoids over the course of cetacean macroevolution (5). Fossils are unrevealing about the origins of the other river dolphins (see references in ref. 5). Pontoporiids appear >11 Myr B.P., represented by marine Brachydelphis from Peru and scattered younger records from marine strata. A possible iniid is known from 10–11 Myr B.P. (55), but reliably identified iniids are younger freshwater species. Origins are uncertain for Lipotes. Our predicted branching times (Fig. 5) would not preclude an odontocete radiation into freshwaters (19) linked to continental flooding caused by high Middle Miocene sea levels.
SINEs corroborate the Platanista lineage as ancient. This genus is the only living member of this once diverse clade. These dolphins now are endangered critically because of human activity. The looming extinction of this unique clade should be a major conservation priority.
Acknowledgments
We thank Drs. Hidehiro Kato, Mutsuo Goto, Hideaki Abe, and Isao Munechika for samples of various animals. We especially appreciate Dr. Tadasu K. Yamada for giving us the opportunity to use samples of Ganges River dolphins, and Drs. Naoki Kohno, Hans Thewissen, and Christian de Muizon for stimulating discussions. This work was supported by a Grant-in-Aid to N.O. and M.H. from the Ministry of Education, Science, Sports and Culture of Japan.
Abbreviations
- SINE
short interspersed elements
- Myr
million years
- B.P.
before present
Footnotes
References
- 1.Flower W H. Proc R Inst Great Britain. 1883;10:360–376. [Google Scholar]
- 2.Van Valen L. Evolution (Lawrence, Kans) 1968;22:37–41. [Google Scholar]
- 3.Barnes L G, Mitchell E D. In: Evolution of African Mammals. Maglio V J, Cooke H S B, editors. Cambridge, MA: Harvard Univ. Press; 1978. pp. 582–602. [Google Scholar]
- 4.Rice D W. Marine Mammals of the World: Systematics and Distribution. Lawrence, KS: Soc. Mar. Mamm.; 1998. [Google Scholar]
- 5.Fordyce R E, Barnes L G. Annu Rev Earth Planet Sci. 1994;22:419–455. [Google Scholar]
- 6.de Muizon C. Bull Mus Natl Hist Nat (Paris) 1991;4 (Series 12 C):279–326. [Google Scholar]
- 7.Heyning J E. Contrib Sci, Nat Hist Mus L A Co. 1989;405:1–64. [Google Scholar]
- 8.Milinkovitch M C, Ortì G, Meyer A. Nature (London) 1993;361:346–348. doi: 10.1038/361346a0. [DOI] [PubMed] [Google Scholar]
- 9.Milinkovitch M C. Trends Ecol Evol. 1995;10:328–334. doi: 10.1016/s0169-5347(00)89120-x. [DOI] [PubMed] [Google Scholar]
- 10.Milinkovitch M C. In: Molecular Genetics of Marine Mammals. Dizon A E, Chivers S J, Perrin W F, editors. Lawrence, KS: Soc. Mar. Mamm.; 1997. pp. 317–338. [Google Scholar]
- 11.Arnason U, Gullberg A. Nature (London) 1994;367:726–728. doi: 10.1038/367726a0. [DOI] [PubMed] [Google Scholar]
- 12.Arnason U, Gullberg A. Mol Biol Evol. 1996;13:407–417. doi: 10.1093/oxfordjournals.molbev.a025599. [DOI] [PubMed] [Google Scholar]
- 13.Messenger S L, Mcguire J A. Syst Biol. 1998;47:90–124. doi: 10.1080/106351598261058. [DOI] [PubMed] [Google Scholar]
- 14.Heyning J E. Mar Mamm Sci. 1997;13:596–613. [Google Scholar]
- 15.Simpson G G. Bull Am Mus Nat Hist. 1945;85:1–350. [Google Scholar]
- 16.de Muizon C. Proc San Diego Mus Nat Hist. 1994;29:135–146. [Google Scholar]
- 17.de Muizon C. Compt Rend Acad Sci, Series 2. 1985;301:359–362. [Google Scholar]
- 18.Cassens I, Vicario S, Waddell B G, Balchowsky H, Van Belle D, Ding W, Fan C, Lal Mohan R S, Simos-Lopes P C, Bastida R, et al. Proc Natl Acad Sci USA. 2000;97:11343–11347. doi: 10.1073/pnas.97.21.11343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton H, Caballero S, Collins A G, Brownell R L. Proc R Soc London Ser B Biol Sci. 2001;268:549–556. doi: 10.1098/rspb.2000.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shedlock A M, Okada N. BioEssays. 2000;22:148–160. doi: 10.1002/(SICI)1521-1878(200002)22:2<148::AID-BIES6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto M M. Curr Biol. 1999;9:R816–R819. doi: 10.1016/s0960-9822(99)80498-9. [DOI] [PubMed] [Google Scholar]
- 22.Murata S, Takasaki N, Saitoh M, Okada N. Proc Natl Acad Sci USA. 1993;90:6995–6999. doi: 10.1073/pnas.90.15.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi K, Terai Y, Nishida M, Okada N. Mol Biol Evol. 1998;15:391–407. doi: 10.1093/oxfordjournals.molbev.a025936. [DOI] [PubMed] [Google Scholar]
- 24.Shimamura M, Yasue H, Ohshima K, Abe H, Kato H, Kishiro T, Goto M, Munechika I, Okada N. Nature (London) 1997;388:666–670. doi: 10.1038/41759. [DOI] [PubMed] [Google Scholar]
- 25.Nikaido M, Rooney A P, Okada N. Proc Natl Acad Sci USA. 1999;96:10261–10266. doi: 10.1073/pnas.96.18.10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanger F, Nicklen S, Coulson A R. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saiki R K, Gelfand D H, Stoffel S, Scharf S J, Higuchi R, Horn G T, Mullis K B, Erlich H A. Science. 1988;239:487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 28.Blin N, Stafford D W. Nucleic Acids Res. 1976;3:2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swofford D L. PAUP*: Phylogenetic Analysis Using Parsimony. Sunderland, MA: Sinauer; 1998. , Version 4.0. [Google Scholar]
- 30.Yang Z H. Comput Appl Biosci. 1997;13:555–556. doi: 10.1093/bioinformatics/13.5.555. [DOI] [PubMed] [Google Scholar]
- 31.Hasegawa M, Kishino H, Yano T. J Mol Evol. 1985;22:160–174. doi: 10.1007/BF02101694. [DOI] [PubMed] [Google Scholar]
- 32.Yang Z H. Trends Ecol Evol. 1996;11:367–372. doi: 10.1016/0169-5347(96)10041-0. [DOI] [PubMed] [Google Scholar]
- 33.Thorne J L, Kishino H, Painter I S. Mol Biol Evol. 1998;15:1647–1657. doi: 10.1093/oxfordjournals.molbev.a025892. [DOI] [PubMed] [Google Scholar]
- 34.Lum J K, Nikaido M, Shimamura M, Shimodaira H, Shedlock A M, Okada N, Hasegawa M. Mol Biol Evol. 2000;17:1417–1424. doi: 10.1093/oxfordjournals.molbev.a026242. [DOI] [PubMed] [Google Scholar]
- 35.Bajpai S, Gingerich P D. Proc Natl Acad Sci USA. 1998;95:15464–15468. doi: 10.1073/pnas.95.26.15464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milinkovitch M C, LeDuc R G, Adachi J, Farnir F, Georges M, Hasegawa M. Genetics. 1996;144:1817–1833. doi: 10.1093/genetics/144.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gatesy J. In: The Emergence of Whales: Evolutionary Patterns in the Origin of Cetacea. Thewissen J G M, editor. New York: Plenum; 1998. pp. 63–111. [Google Scholar]
- 38.de Muizon C. Am Mus Novit. 1987;2904:1–27. [Google Scholar]
- 39.de Muizon C. Ann Paleontol. 1988;74:115–183. [Google Scholar]
- 40.Fordyce R E. Proc San Diego Mus Nat Hist. 1994;29:147–176. [Google Scholar]
- 41.Fraser F C, Purves P E. Bull Br Mus (Nat Hist), Zool. 1960;7:1–140. [Google Scholar]
- 42.Kasuya T. Sci Rep Whales Res Inst (Tokyo) 1973;25:1–103. [Google Scholar]
- 43.Reeves R R, Brownell R L. In: Handbook of Marine Mammals: River Dolphins and the Larger Toothed Whales. Ridgway S H, Harrison R J, editors. Vol. 4. London: Academic; 1989. pp. 69–99. [Google Scholar]
- 44.Barnes L G. Contrib Sci, Nat Hist Mus L A Co. 1985;363:1–34. [Google Scholar]
- 45.Fordyce R E. Special Publication of the Geol Soc London. 1989;47:269–281. [Google Scholar]
- 46.Mitchell E D. Can J Fish Aquat Sci. 1989;46:2219–2235. [Google Scholar]
- 47.Dingle R V, Lavelle M. J Geol Soc. 1998;155:433–437. [Google Scholar]
- 48.Uhen M D. J Paleontol. 1999;73:512–528. [Google Scholar]
- 49.Uhen M D. In: The Emergence of Whales: Evolutionary Patterns in the Origin of Cetacea. Thewissen J G M, editor. New York: Plenum; 1998. pp. 29–61. [Google Scholar]
- 50.Waddell V G, Milinkovitch M C, Berube M, Stanhope M J. Mol Phylogenet Evol. 2000;15:314–318. doi: 10.1006/mpev.1999.0751. [DOI] [PubMed] [Google Scholar]
- 51.Fordyce R E. In: Eocene-Oligocene Climatic and Biotic Evolution. Prothero D, Berggren W A, editors. Princeton: Princeton Univ. Press; 1992. pp. 368–381. [Google Scholar]
- 52.Ivany L C, Patterson W P, Lohmann K C. Nature (London) 2000;407:887–890. doi: 10.1038/35038044. [DOI] [PubMed] [Google Scholar]
- 53.Diester-haass L, Robert C, Chamley H. Mar Geol. 1996;131:123–149. [Google Scholar]
- 54.Salamy K A, Zachos J C. Palaeogeogr Palaeoclimatol Palaeoecol. 1999;145:61–77. [Google Scholar]
- 55.Gottfried M D, Bohaska D J, Whitmore F C. Proc San Diego Soc Nat Hist. 1994;29:229–238. [Google Scholar]
- 56.Morgan G S. Proc San Diego Soc Nat Hist. 1994;29:239–268. [Google Scholar]
- 57.Whitfield P. Encyclopedia of Animals. New York: Simon & Schuster; 1998. pp. 184–193. [Google Scholar]