Abstract
Albendazole, a BCS class II drug, has poor dissolution characteristic. In order to enhance dissolution nanocrystals were prepared by antisolvent precipitation process using PVP K-30(0.05%,0.1%,0.2%,0.4%) as stabilizer. The process was optimized in terms of concentration of stabilizer(PVP K 30) in order to enhance dissolution and obtain stable particles with a small mean particle size. Nanocrystals were characterized with respect to particle size, in vitro dissolution and X-ray diffraction pattern. Decrease in particle size of nanocrystals was observed with increase in concentration of stabilizer. Dissolution of nanocrystals also improved with increase in concentration of PVP K-30. Crystalline state evaluation before and following particle size reduction was conducted through XRD to denote any possible transformation to an amorphous state during process.
KEY WORDS: Albendazole, dissolution enhancement, nanocrystals
Albendazole, a BCS class II drug is a broad-spectrum anthelmintic.[1] It is successfully used in experimental and clinical chemotherapy of different intestinal and systemic parasitosis. Its efficacy is often limited by poor intestinal absorption due mainly to its low aqueous solubility(ca. 0.5mg/L).[2] Efficacy of albendazole can be enhanced by improving its dissolution. Reduction in particle size to nanoscale can help in dissolution improvement by increase in surface area. Aim of the present work is to prepare nanocrystals of albendazole by antisolvent precipitation method. The specific objective is to assess effect of stabilizer (PVP K 30) used for the preparation of nanocrystals on dissolution and particle size of prepared nanocrystals.
Materials and Methods
Materials
Albendazole was obtained as a gift sample from Glaxo smithkline Pharmaceuticals Ltd. Polyvinyl pyrrolidone(PVP K 30) and Dimethyle Sulfoxide (DMSO) were purchased from HiMedia and Merck respectively. All the other reagents were of analytical grade and purchased from Loba chem. and HiMedia.
Preparation of nanocrystals
Nanocrystals were prepared by anti solvent precipitation technique. Albendazole was dissolved in dimethyl sulfoxide. Under constant stirring at 1400 rpm the prepared drug solution was injected drop wise into water containing PVP K 30(0.05%,0.1%,0.2%,0.4%w/v) Immediately, particles precipitated from the antisolvent and a milk-like suspension formed which was than filtered and dried. Drying was carried out using tray dryer at 40°c.
In vitro dissolution
Powder dissolution study was carried out by using USP apparatus II(paddle)(Model: TDT-08L, Make: Electrolab, India), dissolution media was 900 ml of 0.1 N HCl at a temperature of 37±0.5 °C at 50 rpm. 4ml of dissolution medium was withdrawn at regular time intervals (5,10,15,30,45,60,90minutes) and filtered and 4ml of 0.1N HCl was added to jar. Filtered Sample was diluted with 0.1N NaOH. Absorbance of the resultant solution was measured by ultra violet spectrophotometer (Model: UV- 1800 Make: Shimadzu) at 307.8nm using 0.1N NaOH as a blank.
Particle size determination
Particle size was measured by dynamic light scattering technique using particle size analyser (Model: NanoS90 Make: Malvern, USA).
X-ray diffraction study (xrd)
X-ray diffraction analysis was carried out for pure drug and prepared Nanocrystals using X-Ray diffractometer (Model: D2 phaser Make: Bruker, USA). The powder was scanned from 10 to 80° (2θ).
Results and Discussion
Albendazole nanocrystals was prepared using different concentration of PVP K 30 and characterized with respect to particle size, in vitro dissolution and X-ray diffraction pattern. Results of Particle size and %drug release at 30 minute (Q30 min) and 60 minute (Q60 min) for unmilled drug and different batches of albendazole nanocrystals were described in Table 1. Dissolution profile and XRD pattern of unmilled drug and Prepared Albendazole nanocrystals were shown in Figures 1 and 2 respectively.
Table 1.
Particle size and %drug release at 30 minute (Q30 min) and 60 minute (Q60 min) for unmilled drug and different batches of albendazole nanocrystals
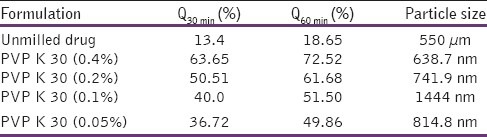
Figure 1.
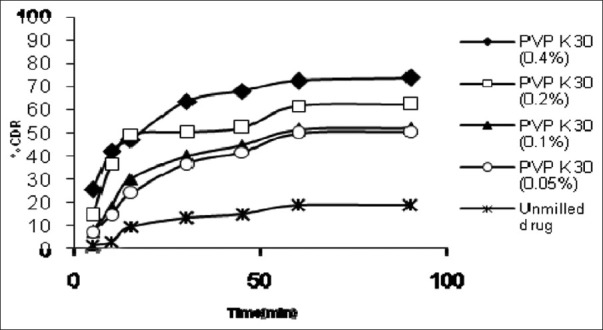
Dissolution profile of unmilled drug and different batches of Albendazole nanocrystals
Figure 2.
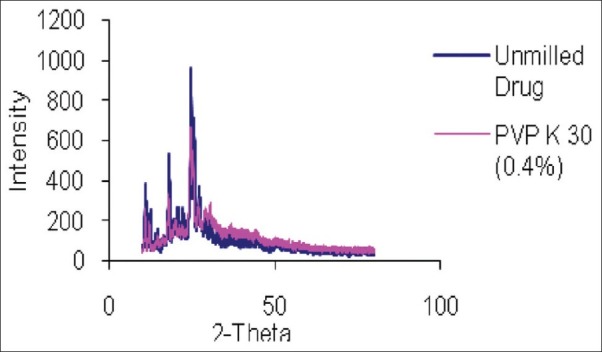
XRD pattern of Unmilled drug and Albendazole nanocrystals (PVP K 30 (0.4%))
Conclusion
Nanocrystals produced using PVP K 30 showed high dissolution rate as compared to unmilled drug. Enhancement of dissolution rate observed with increase in PVP K 30 concentration may be attributed to reduction in particle size and improvement in wetting of particles. Improved dissolution can also be attributed to reduction in crystallinity. Comparison of X-Ray diffraction pattern of unmilled drug and nanocrystals indicate that for nanocrystals reduction in peak intensity takes place but peak position remains the same. Physical stability of nanocrystals on accelerated storage conditions need to be assessed.
Acknowledgement
Glaxo Smithkline Pharmaceuticals Ltd for gift sample of Albendazole.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Torrado S, Lopez ML, Bolas F, Cadorniga R. A novel formulation of albendazole solution: Oral bioavailability and efficacy evaluation. International Journal of Pharmaceutics. 1997;156:181–7. [Google Scholar]
- 2.Yepez, Mulia LN. Evaluation of Albendazole Prodrugs in Experimental Trichinellosis. Archives of Medical Research. 1999;30:368–74. doi: 10.1016/s0188-0128(99)00054-8. [DOI] [PubMed] [Google Scholar]


