Abstract
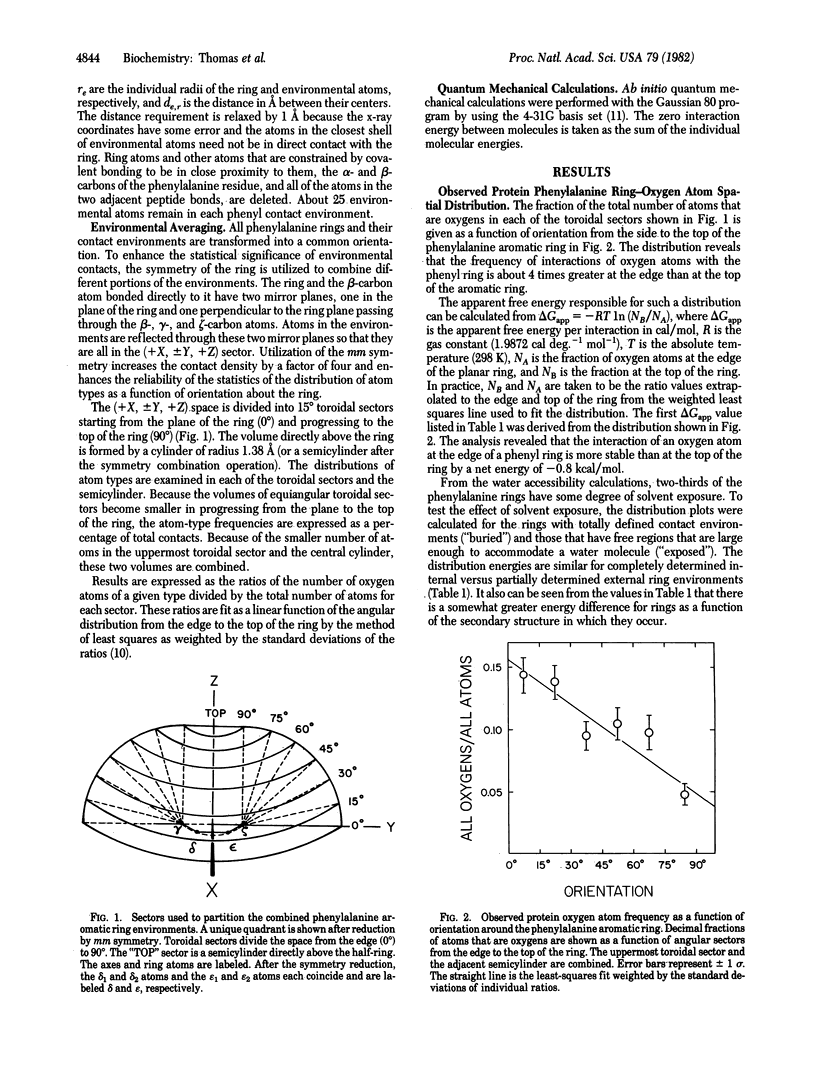
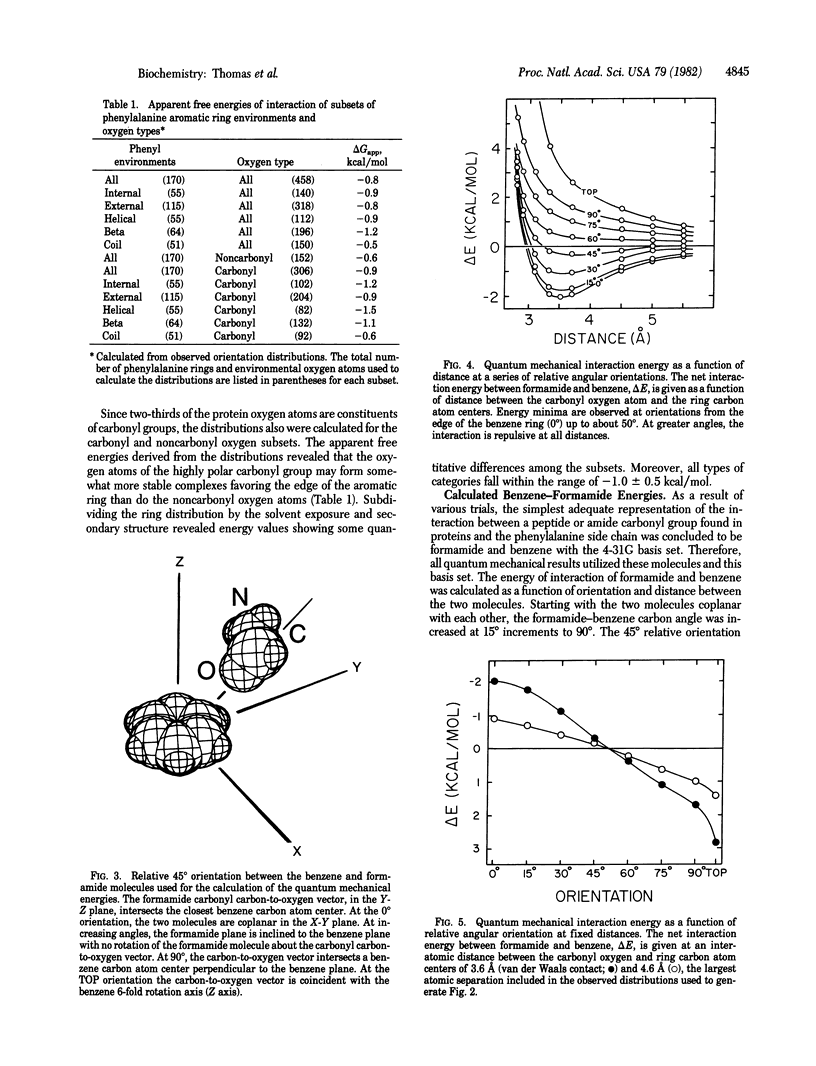
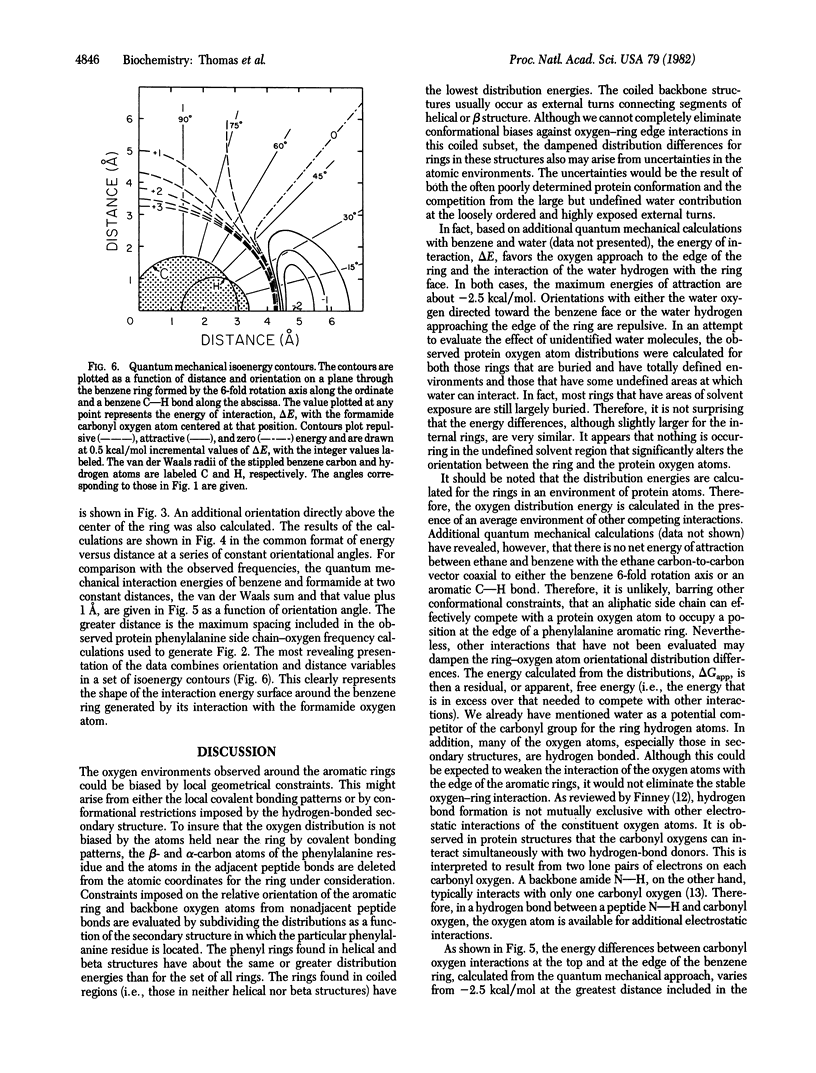
The atomic environments of 170 phenylalanine-residue aromatic rings from 28 protein crystal structures are transformed into a common orientation and combined to calculate an average three-dimensional environment. The spatial distribution of atom types in this environment reveals a preferred interaction between oxygen atoms and the edge of the planar aromatic rings. From the difference in frequency of interaction of oxygen atoms with the edge and the top of the ring, an apparent net free energy difference of interaction favoring the edge of the ring is estimated to be about -1 kcal/mol (1 cal = 4.184 J). Ab initio quantum mechanical calculations, performed on a model consisting of benzene and formamide, indicate that the observed geometry is stabilized by a favorable enthalpic interaction. Although benzene rings are considered to be nonpolar, the electron distribution is a complex multipole with no net dipole moment. The observed interaction orientation frequencies demonstrate that these multipolar electron distributions, when occurring at the short distances encountered in densely packed protein molecules, are significant determinants of internal packing geometries.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Finney J. L. Volume occupation, environment and accessibility in proteins. The problem of the protein surface. J Mol Biol. 1975 Aug 25;96(4):721–732. doi: 10.1016/0022-2836(75)90148-5. [DOI] [PubMed] [Google Scholar]
- Finney J. L. Volume occupation, environment, and accessibility in proteins. Environment and molecular area of RNase-S. J Mol Biol. 1978 Mar 5;119(3):415–441. doi: 10.1016/0022-2836(78)90223-1. [DOI] [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H., Nockolds C. E. Carp muscle calcium-binding protein. II. Structure determination and general description. J Biol Chem. 1973 May 10;248(9):3313–3326. [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Nockolds C. E., Kretsinger R. H., Coffee C. J., Bradshaw R. A. Structure of a calcium-binding carp myogen. Proc Natl Acad Sci U S A. 1972 Mar;69(3):581–584. doi: 10.1073/pnas.69.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace C. N. The stability of globular proteins. CRC Crit Rev Biochem. 1975 May;3(1):1–43. doi: 10.3109/10409237509102551. [DOI] [PubMed] [Google Scholar]
- Richards F. M. The interpretation of protein structures: total volume, group volume distributions and packing density. J Mol Biol. 1974 Jan 5;82(1):1–14. doi: 10.1016/0022-2836(74)90570-1. [DOI] [PubMed] [Google Scholar]
- Shrake A., Rupley J. A. Environment and exposure to solvent of protein atoms. Lysozyme and insulin. J Mol Biol. 1973 Sep 15;79(2):351–371. doi: 10.1016/0022-2836(73)90011-9. [DOI] [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. C. Theoretical models for the mechanism of denaturation. Adv Protein Chem. 1970;24:1–95. [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]


