Abstract
The objective of this study was to increase the oral bioavailability of Raloxifene having an absolute bioavailability only 2% due to extensive first pass hepatic metabolism by incorporating it in Solid Lipid Nanoparticles (SLNs). The optimized RSLNs prepared by Ultrasonic Emulsification and Low Temperature Solidification method showed the mean particle size, zeta potential and percentage drug entrapment of 101.4±3.5 nm, 19.4±0.279 mv, 97.67±1.02% respectively. The in-vitro intestinal permeability study indicated significantly higher permeation of the RSLNs than the marketed preparation. The in-vivo studies showed that pharmacokinetic parameters for the RSLNs were 3.5 times higher than the marketed preparation indicating significant increase in the oral bioavailability of the Raloxifene.
KEY WORDS: Drug entrapment, estrogen, solvent emulsification, zeta potential
Raloxifene is a second generation Selective Estrogen Receptor Modulator (SERM) used to prevent osteoporosis in post menopausal women. It is administered in the form of a tablet having strength 60 mg, as a single daily dose. Oral absorption of the drug is about 60%, but absolute bioavailability is only 2%. This is because of extensive first pass hepatic metabolism. The metabolite formed is generally excreted in the feces. In the present study, we have tried to incorporate Raloxifene in SLNs and thereby increase its bioavailability and decrease its side effects.
Materials and Methods
Materials
Raloxifene was obtained as a gift sample from Aarti Drugs Ltd., Mumbai. Dynasan 116 (Glyceryl Tripalmitrate) was a gift from Colorcon Asia Pvt. Ltd., Goa, India. Tween 80 and PEG 400 were generous gift from S.D. Fine Chemicals, Mumbai. Distilled water was freshly prepared in the laboratory of the institute.
Methods
Selection of lipids was done on the basis of the partition co-efficient and selection of surfactant and co surfactant was done on the basis of Particle Size and Percentage Drug Entrapment obtained.[1]
Preparation of Raloxifene loaded solid lipid nanoparticles
Ultrasonic – solvent emulsification and low temperature solidification method
Accurately weighed required quantities of Surfactant and Co – surfactant were dissolved in Distilled water. The lipids were melted and Raloxifene was dispersed in it. Both the phases were warmed 5°C above the melting point of the lipid. Then the dispersion phase was gently poured in to hot lipid phase. The mixture was then subjected to high speed stirring using Ultra Turrex for several minutes. The dispersion obtained was probe sonicated for different cycles. On cooling the dispersion, up to room temperature, the lipids which are solid at room temperature were recrystallized and thereby formed SLNs. SLNs were then subjected to lyophillization for 24 hrs.
Characterization of RSLNS
The prepared SLNs were characterized and optimized for Particle Size, Percentage Drug Entrapment, Zeta Potential, DSC, and SEM. Stirring speed and Time of Rotation were optimized as process parameters.[2,3]
Results and Discussion
At the end of partition – coefficient study, the maximum amount of drug partitioned was found with Dyanasan – 116 (Glyceryl Tripalmitate). Its concentration selected was 4.4%, as it gave minimum particle size and maximum percentage drug entrapment. As far as surfactant and co-surfactant are considered, Tween 80 (with concentration of 2.6%) was selected as surfactant and the PEG-400 as co – surfactant (2%), as they gave lower particle size and higher percentage drug entrapment.
The stirring speed and the time of rotation were optimized upto 9500 RPM and 7 minutes respectively, as it gave an average particle size of 103.1 + 1.553 nm and % drug entrapment of 96.78 + 1.172. The number of cycles for sonication was optimized to 3. Mean particle size of optimized SLNs was found to be 101.4 + 3.569 nm with the PDI of 0.203 + 0.064. The small size of SLNs is expected to contribute to the increment of oral bioavailability of Raloxifene. The small value of Poly Dispersity Index (PDI) indicates that the particles are closed to monodisperse. Zeta Potential of the optimized SLNs was found to be 19.4 + 0.279 mV, which may be attributed to the cationic nature of the lipid matrix. The percentage drug entrapment of the optimized batch was 97.67 + 1.021. The high percentage drug entrapment was due to the lipophilic nature of the drug. The DSC thermogram of Raloxifene loaded SLNs [Figure 1] showed endothermic peak of melting at 45°C for lipid and surfactant respectively. This thermogram does not show the endothermic peak of drug melt, which indicates that the physical state of Raloxifene has changed from crystalline to amorphous, and thereby it can be dispersed homogenously in matrix. SEM image [Figure 2] shows that the Raloxifene SLNs are discrete, spherical, and regular in shape. The surface is smooth indicating that drug is not present as crystals on the surface but is entrapped within the lipid matrix.
Figure 1.
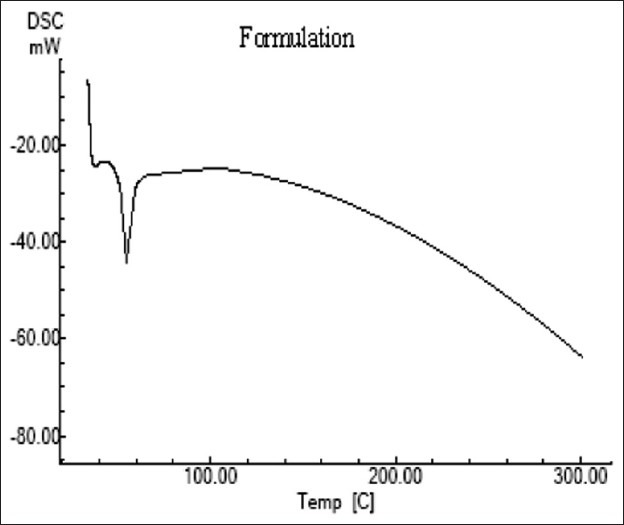
DSC thermogram of RSLNs
Figure 2.

SEM Image of RSLNs
The Pharmacokinetic studies were performed in Male wistar rats weighing about 250 – 300 g. As apparent from the results tabulated in Table 1, the Cmax of the Raloxifene loaded SLNs (RSLNs) was found to be 3.5 times higher than the marketed preparation. The t1/2 of the RSLNs had increased remarkably than that of marketed preparation.
Table 1.
Pharmacokinetic parameters
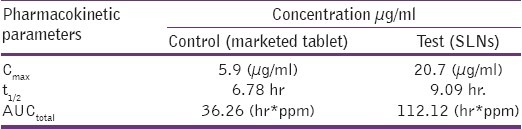
This indicates that the absorption of the drug by this formulation was found to be much higher than that of commercially available tablets. The reasons of increased bioavailability include bypass of the first pass hepatic metabolism because of preferential uptake of RSLNs by lymphatic system as compared to the systemic circulation. The surfactant increases the permeation by reducing the surface tension and the presence of PEG in the formulation increases circulation time of the formulation, and thereby contributes to the increase in bioavailability.
The stability study performed as per ICH Guidelines showed that the particle size and zeta potential of the RSLNs shows little changes when stored at room temperature as well as at refrigeration temperature (2–8°C) indicating that the preparation is stable at room temperature.
Conclusion
The Raloxifene loaded SLNs prepared by Ultrasonic Solvent Emulsification and Low-Temperature Solidification Method, using Dyanasan 116 (Glyceryl Tripalmitate) as lipid matrix, Tween 80 as surfactant and PEG 400 as co-surfactant were found to be stable. Comparison of pharmacokinetic parameters of the SLNs with marketed preparation showed that SLNs improves the bioavailability of a drug to a significant extent. This increase in the bioavailability can lead to reduction in the dose required for therapeutic action and frequency of administration of the drug. This can lead to fewer side effects and overall decrease in the cost of therapy. Thus it can be concluded that the Solid Lipid Nanoparticles are suitable carriers for improving the oral bioavailability of Raloxifene.
Acknowledgments
We acknowledge the generous donation of Raloxifene by Aarti Drugs Ltd. Mumbai, Dynasan 116 donated by Colorcon Asia Pvt. Ltd., Goa and Tween 80 and PEG 400 by S. D. Fine Chemicals Mumbai.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Zurmuhlen C, Mehnert SW. Solid lipid nanoparticles (SLN) for controlled drug delivery-drug release and release mechanism. Eur. J. Pharm. Biopharm. 1998;45(2):149–155. doi: 10.1016/s0939-6411(97)00150-1. [DOI] [PubMed] [Google Scholar]
- 2.Mehnert W, Mader K. Solid lipid nanoparticles: production, characterization and applications. Adv. Drug Deliv. Rev. 2001;47(2-3):165–196. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 3.Mehnert SW, Lucks JS, Müller RH. Solid lipid nanoparticles (SLN) for controlled drug delivery: Production, characterization and sterilization. J. Control. Release. 1994;30(1):83–96. [Google Scholar]


