Abstract
The objective of present work was to prepare microspheres of montelukast sodium using a natural polymer- chitosan by spray drying method by using glutaraldehyde as a cross linking agent. The microspheres were characterized for size, shape, dissolution, swelling and mucoadhesion. It was observed that, all microspheres were spherical in shape with narrow size distribution. Microspheres had mean particle size of 7-12 μm, with % encapsulation efficiency of 78-86%. The % yield was 32-49% and drug load was 48-53%. With the increase in proportion of chitosan in formulation mucoadhesive strength was increase and also increased in particle size of microspheres. As the drug:polymer ratio increase drug loading was increase and % encapsulation efficiency was also increase.
KEY WORDS: Chitosan, gluteraldehyde, microspheres, montelukast sodium, mucosdhesion, spray dryer
Microspheres and nanoparticles have been explored as drug delivery carrier to administration of drug agents used in pulmonary disorders. Dry powder inhalers have edge over the existing liquid inhaler systems like Metered dose inhalers (MDIs), Nebulizer and Aerosol Sprays etc. A dry powder inhaler (DPI) offer better patient compliance as well as overcomes the instability. Controlled release dosage forms provide advantage over immediate release dosage form: reduction in side effects, first pass metabolism, maintenance of effective plasma concentration and reduced dosing frequency. There are many advantages to develop sustained release formulations for pulmonary delivery of drugs for localized treatment of diseases like asthma, chronic obstructive pulmonary disease and infectious diseases. Asthma is a chronic inflammatory disease of the airways, characterized by hyper responsiveness to a variety of stimuli. The montelukast sodium is a leukotrine receptor antagonist (LTRA) used for the maintenance treatment of asthma, chronic asthma attacks and to relive symptoms of seasonal allergies. The main drawback of conventional montelukast formulation is that it undergoes hepatic first pass metabolism. Thus, it shows plasma or biological half-life 2.5 to 5.5 hrs, thereby decreasing bioavailability up to 64%. The present work describes such delivery system, which will improve the bioavailability of montelukast na. and decrease dosing frequency. Spray drying is the continuous transformation of liquid feed into dried particulate form by spraying the feed into a hot drying medium. When preparing a formulation suitable for DPI, micronization is usually applied to reduce particle size of drug powder to less than 5 μm, however it shows strong interparticular cohesion leading to poor flow properties. So to improve these properties spray drying is use as alternative approach.
Materials and Methods
Montelukast sodium obtained as a gift sample from Cedilla health care, Moriya, chitosan (medium. molecular weight), mannitol A. R., propylene glycol A. R., leucine, glacial acetic acid A. R., distill water, ethanol, gluterladehyde.
Elemental analysis was used to determine the degree of deacetylation in raw chitosan sample with CHNS/O Elemental Analyzer (model name PerkinElmer PE 2400 series 2).
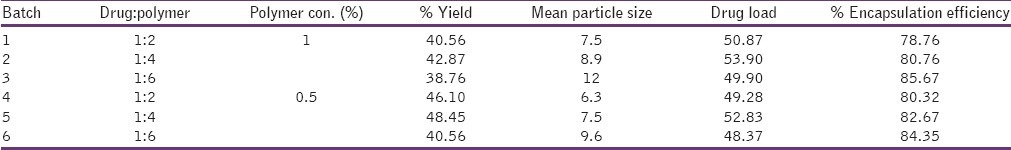
Table 1.
Formulation
Microspheres were prepared by spray dried technique. The chitosan-Drug solution was spray dried by Lab-ultima spray drier (LU-222 advanced, Mumbai, India) with standard 0.7 mm nozzle. The spray drying conditions such as inlet temperature, outlet temperature, pump prate, pressure and aspirator setting were set as 130°C, 70-80°C, 3 ml/min, 2 kg/cm2 and 50 m3/h, respectively. The prepared microspheres were characterized for Loading efficiency, entrapment efficiency, morphological characterization by scanning electron microscope, swelling index determination and An in vitro mucoadhesive property of dry powder was performed by modified liquid falling film method.
Results and Discussions
Degree of deacetylation of chitosan was found to be 90%, the percent yield, mean particle size, drug loading efficiency and encapsulation efficiency was found to be 32-49%, 7-12 μm, 48-53% and % 78-86% respectively. SEM micrographs revealed that most of the spray dried microspheres were spherical in shape. Surface of microspheres were found to be smooth. Based on swelling index determination results, as the concentration of polymer increased, the swelling of microspheres were found to be increased because the polymer network increases with increasing in the concentration of polymer. In vitro drug release study revealed that the burst release of drug was observed in this study indicating approximately 50-60% of drug release within first 30 minutes. The sustained drug release was seen after two hours which followed zero order release pattern, more than 90% of drug released in 8 hours measure in U. V. at 285 nm. In vitro mucoadhesive study indicated that, more than 50% of microspheres were adhering to rat intestine that attributed with the increasing the mucoadhesion by increasing the drug: polymer ratio.
Conclusion
The recent studies on spray dried chitosan microspheres demonstrated possibility of generating highly respirable powders that exhibits good aerosolization properties. Effective microencapsulation and loading of montelukast na. with controlled size was feasible with spray drying technique. It is possible to produce good spherical particles with size less than 10 μm with spray drying technique. The applied processing conditions resulted in drug loaded chitosan microspheres having good surface properties and spherical shape suitable for inhalation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Janugade BU, Patil SS, PATIL SV, Lad IJCRGG. 2009;1(3):690–695. [Google Scholar]
- 2.A Misra, P Muttil, J Kaur, K Kumar, A. B Yadav, R Sharma. Eur. J. of Pharm. Sci. 2007;32:140–150. [Google Scholar]
- 3.B. K Kim, SJ Hwang, JB Park, HJ Park. J. of Microencapsule. 2002;19:811. [Google Scholar]
- 4.D. O Corrigan, O. I Corrigan, A. M Healy. Int. J. Pharm. 2006;322:22–30. [Google Scholar]
- 5.Y. C Huang, C. H Chiang, M. K Yeh. J. Microencapsul. 2003;20(2):247–260. [PubMed] [Google Scholar]


