Abstract
Uteroglobin is a small steroid-binding protein that is differentially regulated by steroid hormones in several tissues of the rabbit. In endometrium, the levels of uteroglobin mRNA increase after progesterone administration due to an enhanced rate of transcription of the uteroglobin gene. As a prerequisite for understanding the molecular mechanisms that modulate uteroglobin gene expression, we have isolated and characterized the uteroglobin gene. We first synthesized, cloned, and sequenced a uteroglobin cDNA that was used to screen a rabbit gene library and to show that the uteroglobin gene is not reiterated in the rabbit genome. We obtained three recombinant phages containing uteroglobin gene sequences and covering 35 kilobases of the rabbit genome. The uteroglobin gene is 3 kilobases long and is composed of three short exons separated by a long and a short intron. The complete coding sequence, the short intron, part of the large intron, and the flanking sequences have been subjected to sequence analysis. The salient features of the nucleotide sequence, including the absence of a canonical "T-A-T-A box," are discussed. A possible relationship is considered between the exon-intron structure of the gene and the known structure and function of uteroglobin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnemann J., Heins B., Beato M. Synthesis and characterization of a DNA complementary to pre-uteroglobin mRNA. Eur J Biochem. 1979 Sep;99(2):361–367. doi: 10.1111/j.1432-1033.1979.tb13264.x. [DOI] [PubMed] [Google Scholar]
- Atger M., Atger P., Tiollais P., Milgrom E. Cloning of rabbit genomic fragments containing the uteroglobin gene. J Biol Chem. 1981 Jun 25;256(12):5970–5972. [PubMed] [Google Scholar]
- Atger M., Mercier J. C., Haze G., Fridlansky F., Milgrom E. N-terminal sequences of uteroglobin and its precursor. Biochem J. 1979 Mar 1;177(3):985–988. doi: 10.1042/bj1770985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atger M., Perricaudet M., Tiollais P., Milgrom E. Bacterial cloning of the rabbit uteroglobin structural gene. Biochem Biophys Res Commun. 1980 Apr 29;93(4):1082–1088. doi: 10.1016/0006-291x(80)90599-9. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Pictet R. L., Rutter W. J., Cordell B., Tischer E., Goodman H. M. Sequence of the human insulin gene. Nature. 1980 Mar 6;284(5751):26–32. doi: 10.1038/284026a0. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra T., Bullock D. W., Woo S. L. Hormonally regulated mammalian gene expression: steady-state level and nucleotide sequence of rabbit uteroglobin mRNA. DNA. 1981;1(1):19–26. doi: 10.1089/dna.1.1981.1.19. [DOI] [PubMed] [Google Scholar]
- Chandra T., Woo S. L., Bullock D. W. Cloning of the rabbit uteroglobin structural gene. Biochem Biophys Res Commun. 1980 Jul 16;95(1):197–204. doi: 10.1016/0006-291x(80)90724-x. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Grez M., Land H., Giesecke K., Schütz G., Jung A., Sippel A. E. Multiple mRNAs are generated from the chicken lysozyme gene. Cell. 1981 Sep;25(3):743–752. doi: 10.1016/0092-8674(81)90182-3. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heins B., Beato M. Hormonal control of uteroglobin secretion and preuteroglobin mRNA content in rabbit endometrium. Mol Cell Endocrinol. 1981 Feb;21(2):139–150. doi: 10.1016/0303-7207(81)90051-4. [DOI] [PubMed] [Google Scholar]
- Klein R. D., Selsing E., Wells R. D. A rapid microscale technique for isolation of recombinant plasmid DNA suitable for restriction enzyme analysis. Plasmid. 1980 Jan;3(1):88–91. doi: 10.1016/s0147-619x(80)90037-2. [DOI] [PubMed] [Google Scholar]
- Maizels N. Dictyostelium 17S, 25S, and 5S rDNAs lie within a 38,000 base pair repeated unit. Cell. 1976 Nov;9(3):431–438. doi: 10.1016/0092-8674(76)90088-x. [DOI] [PubMed] [Google Scholar]
- Malsky M. L., Bullock D. W., Willard J. J., Ward D. N. Progesterone-induced secretory protein. NH2-Terminal sequence of pre-uteroglobin. J Biol Chem. 1979 Mar 10;254(5):1580–1585. [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mirault M. E., Goldschmidt-Clermont M., Artavanis-Tsakonas S., Schedl P. Organization of the multiple genes for the 70,000-dalton heat-shock protein in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5254–5258. doi: 10.1073/pnas.76.10.5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mornon J. P., Fridlansky F., Bally R., Milgrom E. X-ray crystallographic analysis of a progesterone-binding protein. The C2221 crystal form of oxidized uteroglobin at 2.2 A resolution. J Mol Biol. 1980 Mar 15;137(4):415–429. doi: 10.1016/0022-2836(80)90166-7. [DOI] [PubMed] [Google Scholar]
- Müller H., Beato M. RNA synthesis in rabbit endometrial nuclei. Hormonal regulation of transcription of the uteroglobin gene. Eur J Biochem. 1980 Nov;112(2):235–241. doi: 10.1111/j.1432-1033.1980.tb07199.x. [DOI] [PubMed] [Google Scholar]
- Norgard M. V., Keem K., Monahan J. J. Factors affecting the transformation of Escherichia coli strain chi1776 by pBR322 plasmid DNA. Gene. 1978 Jul;3(4):279–292. doi: 10.1016/0378-1119(78)90038-0. [DOI] [PubMed] [Google Scholar]
- Ponstingl H., Nieto A., Beato M. Amino acid sequence of progesterone-induced rabbit uteroglobin. Biochemistry. 1978 Sep 19;17(19):3908–3912. doi: 10.1021/bi00612a003. [DOI] [PubMed] [Google Scholar]
- Popp R. A., Foresman K. R., Wise L. D., Daniel J. C., Jr Amino acid sequence of a progesterone-binding protein. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5516–5519. doi: 10.1073/pnas.75.11.5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Apr;3(4):863–877. doi: 10.1093/nar/3.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra A., Beato M. Influence of chemical modifications of amino acid side chains on the binding or progesterone to uteroglobin. J Steroid Biochem. 1980 Nov;13(11):1347–1353. doi: 10.1016/0022-4731(80)90096-5. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
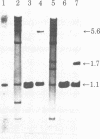
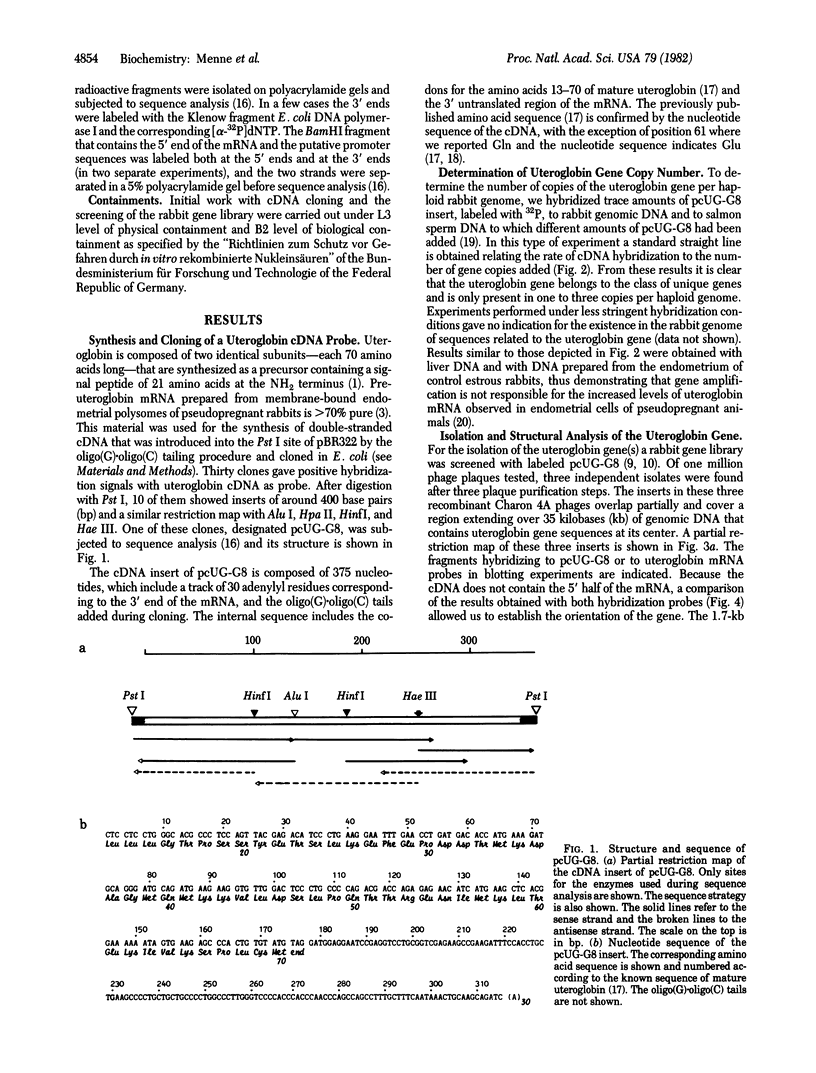
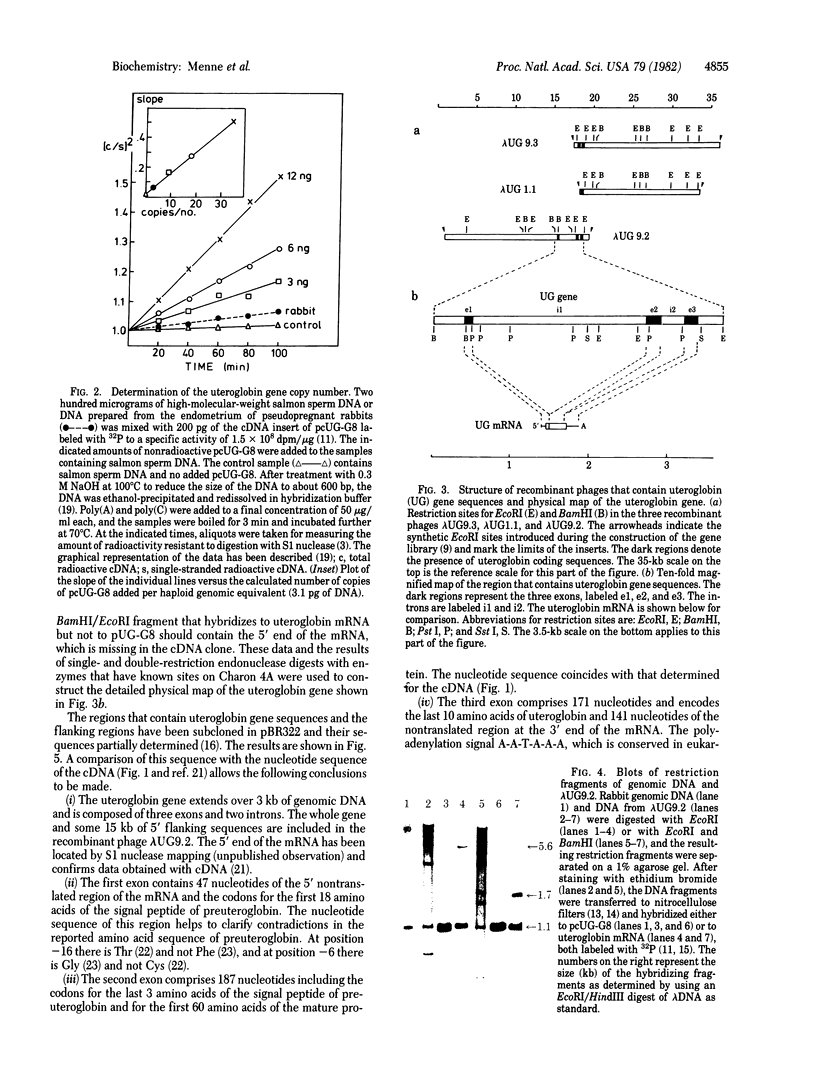
- Snead R., Day L., Chandra T., Mace M., Jr, Bullock D. W., Woo S. L. Mosaic structure and mRNA precursors of uteroglobin, a hormone-regulated mammalian gene. J Biol Chem. 1981 Nov 25;256(22):11911–11916. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tancredi T., Temussi P. A., Beato M. Interaction of oxidized and reduced uteroglobin with progesterone. Eur J Biochem. 1982 Feb;122(1):101–104. doi: 10.1111/j.1432-1033.1982.tb05853.x. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]