Abstract
Artemether and Lumefantrine capsules are indicated for the treatment of P. falciparum malaria cases resistant to both chloroquine and sulphadoxine, pyrimethamine combination. Both artemether and lumefantrine act as blood schizontocides. Artemether is a sesquiterpene lactone derived from artemisinin. Artemisinin is a compound derived from the sweet wormwood plant and has been used for centuries in traditional Chinese medicine to treat fever. Lumefantrine is a synthetic aryl-amino alcohol antimalarial (quinine, mefloquine and halofantrine are members of the same group). Artemether is absorbed fairly rapidly with peak plasma concentrations reached about 2 hours after dosing. Absorption of lumefantrine, a highly lipophilic compound, starts after a lag period of up to 2 hours, with peak plasma concentration about 6-8 hours after dosing. In order to overcome this problem, we have observed that when the drug is given in the soft gelatin dosage form, the bioavailability of the drug is increased. Thus, increasing the absorption of the drug and peak plasma concentration is reached earlier then the conventional dosage form.
KEY WORDS: Artemether, lumefantrine
Formulation development
Preparation of Fill Medicament Composition: Different Fill medicament compositions are developed and shown in Table 1.
Table 1.
Optimized fill formulations
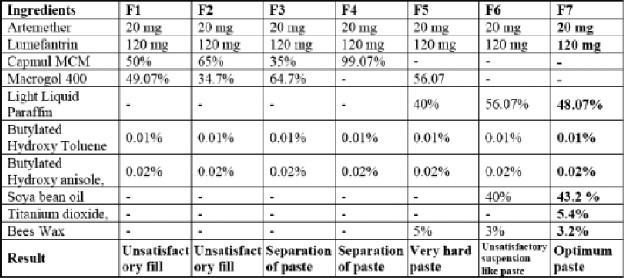
Artemether + Lumefantrine (Artemether + Lumefantrin) as Tablet in the market but in soft gelatin formulation have gained wide popularity and acceptance due to their easier mode of administration and due to greater stability and bioavailability from a manufacturer's point of view. Our objective was mainly to develop a SGC of Artemether + Lumefantrin in order to capture the market of malaria. The main challenge presented to us in the formulation of SGCs of Artemether + Lumefantrin was to obtain a substantially stable and suspension like fill solution. This was due to the fact that Lumefantrin is a potent hydrophobic drug. Ideally formulation is preceded by Preformulation studies of drugs, analytical methods, optimization of formulation and processing variables. The Artemether + Lumefantrin ideally conformed to the standard as specifications. The formulation of SGCs of Artemether + Lumefantrine was carried out in three steps i.e optimization of Fill solution, optimization of the Gelatin Paste for shell formation and encapsulation. Seven different combinations of fill suspensions are given in Table 1 of which only F7 produced stable and optimized fill suspension. However F7 was considered as optimized formulation in terms of its composition and was adopted for final batch processing.
Light Liquid Paraffin, Soya bean oil, Bees Wax, Titanium dioxide, Butylated Hydroxy anisole, Butylated Hydroxy Toluene, Miglyol, Capmul MCM, Macrogol 400 were studied alone and in combinations in order to develop a vehicle to solubilize the hydrophobic Lumefantrin and oil soluble Artemether. The next step involved was formulation of ideal gelatin paste for formulation of gelatin shell which would resist the cross linking of gelatin by the fill contents.
Preparation of gelatin shell composition
The Gelatin mass were prepared and used for encapsulation. The formulae of various compositions are as shown in Table 2.
Table 2.
Gelatin shell compositions
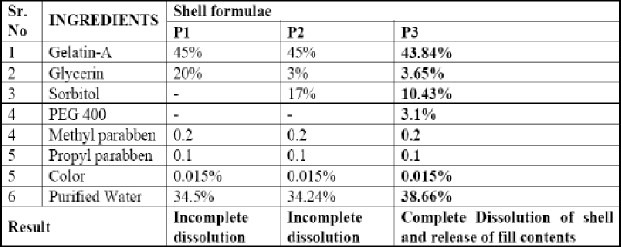
Formulations F7 was encapsulated in to soft gelatin capsule with gelatin composition P3 and evaluated.
Evaluation of artemether+lumefantrine soft gelatin capsules
Uniformity of fill weight of the capsules
Weigh an intact capsule. Open it without losing any part of the shell and remove the contents as completely as possible. Wash the shell with a suitable solvent and keep aside until the odor of the solvent is not perceptible. Weigh the shell. The difference between the weighing gives the weight of the contents. Repeat the procedure with another 19 capsules. The weight complies as per the Pharmacopoeial limits.
Loss on drying of the capsule shell
Soft gelatin capsule shells normally contain 6% to 13% of water and the Loss on Drying of the shell was found to be within the limit.
Disintegration test for soft gelatin capsules
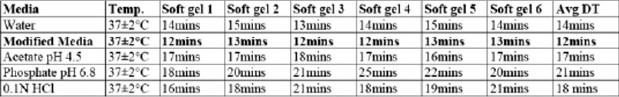
Although there are no official limits specified, ideally a disintegration time within 30 minutes is considered ideal for soft gelatin capsules. The formulated capsules disintegrated within a maximum of about 25 min. as shown in the Table No. 3.
Table 3.
Disintegration test
Dissolution studies of artemether+lumefantrine soft gelatin capsules
Assay
Experimental chromatographic conditions
Stationary phase: Phenomex, 5 μm, C18 (250×4.6 mm) column
Mobile phase: Buffer (1.36 gm Potassium Dihydrogen Ortho phosphate in 900 ml HPLC grade Water pH 3.00Adjust with Orthophosphoric acid): Acetonitrile
Solvent ratio: 40:60 v/v
Detection wavelength: 210 nm
Flow rate: 1.5 ml/minute
Operating pressure: 170 kgf
Run time: 20 mins.
Temperature: Room temperature.
Preparation of standard solution
120 mg of Lumefantrine and 20 mg of Artemether and diluted with the Mobile phase up to 100 ml (Stock Solution).
Aliquots of standard solutions containing 96 μg/ml of Lumefantrine and 16 μg/ml of Artemether diluted with mobile phase.
Figure 1.
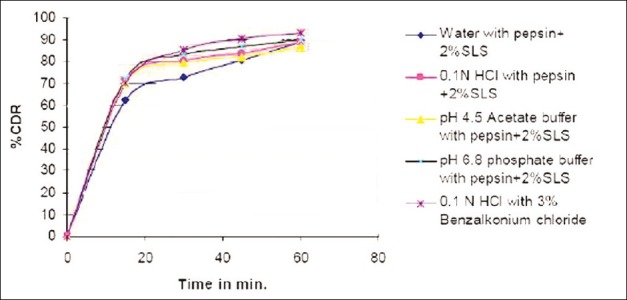
Optimization of dissolution media
Figure 2.
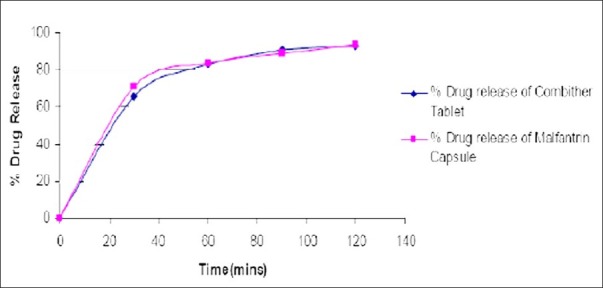
Comparative dissolution studies of developed formula and marketed product
Preparation of sample solution
20 tablets → Average weight → Powdered and weighed a quantity equivalent to 120 mg of Lumefantrine and 20 mg of Artemether transferred to 100 ml standard flask and make up with the with mobile phase. Filter with Whatman filter paper.
Aliquots of solution containing 96 μg/ml of Lumefantrine and 16 μg/ml of Artemether diluted with mobile phase.
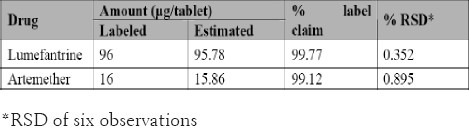
Analysis of formulation
Summary
The most important aspect of this invention is converting two drugs i.e. Artemether and Lumefantrine which are solids at room temperature into a liquid micro suspension and ensuring that this suspension remains homogeneous throughout the process of encapsulation. This is so vital because any kind of heterogeneity will result in non uniform dosage which in turn will give rise to low concentration or high concentration of medicine in the Softgel and both such situations are dangerous to the patient especially because both these drugs are highly potent. A low concentration will result in the organism namely P. falciparum develop drug resistance than optimum concentration will result in side effects. Therefore the choice of Excipient and their optimum concentration in the formula has been discovered and this is the inventive part of this research work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Isabela da Costa César*, Gerson Antônio Pianetti Quantitation of artemether in pharmaceutical raw material and injections by high performance liquid chromatography. Brazilian Journal of Pharmaceutical Sciences. 2009 Oct-Dec;45(4) [Google Scholar]
- 2.Sridhar1 B, et al. A Validated Reverse Phase Hplc Method for the Simultaneous Estimation of Artemether and Lumefantrine in Pharmaceutical Dosage Forms An International Journal of Advances In Pharmaceutical Sciences. 2010 Sep-Oct;1(1) [Google Scholar]


