Abstract
Mucoadhesive drug delivery systems are those that provide intimate contact of the drug with the mucosa for an extended period of time. In present work, mucoadhesive chitosan microspheres of Levosalbutamol sulphate were prepared by Spray drying method. Formulations were characterized for various physicochemical attributes size, encapsulation efficiency, swelling ability, in vitro release study and mucoadhesion study by rat ileum. Through these parameters we conclude that the batch B2 was found to be best mainly by mucoadhesion study and in vitro drug release. Mucoadhesion was found to be increased with incresed concentration of polymer and visa versa in case of drug release. Batch B3 had also similar results with that of Batch B2. That's why here Batch B2 was said to be the best batch with less polymeric content as compare to Batch B3.
KEY WORDS: Chitosan microspheres, spray drying, in vitro release study
Pulmonary drug delivery is an important research area which impacts the treatment of illnesses including asthma, chronic obstructive pulmonary disease and various other diseases. Inhalation gives the most direct access to drug target. Mucoadhesive drug delivery systems are those that provide intimate contact of the drug with the mucosa for an extended period of time. This system utilizes bioadhesion of certain polymers that become adhesive on hydration. The aim of this study was to prepare the pulmonary drug delivery system based on chitosan microspheres loaded with Levosalbutamol sulphate. The objective of the study is to reduce the dosing frequency of conventional dosage form and thus improve patient compliance.
Subjects and Methods
Preparation of Microspheres: Different ratio for drug to polymer was taken. 0.2% of Chitosan S was dispersed in 1% acetic acid solution and then drug was added.
Evaluation parameters
Characterizations by Fourier transform infrared spectrometry (FTIR), Optical microscopy, Swelling studies, Mucoadhesion properties, Determination of drug loading, in-vitro release studies, Statistical analysis.
Results and Discussion
Characterizations by Fourier transform infrared spectrometry
To confirm the interaction of Levosalbutamol sulphate with chitosan, samples were analyzed by Fourier transform infrared spectrometry (FT-IR). Levosalbutamol sulphate with principal splitting peaks at 1600 cm-1 indicating the presence of –NH group. OH group reveals the peak in the region of 3580–2300 cm- 1. Figure 1 shows the FT-IR spectra of Drug + chitosan Physical mixture and drug/chitosan microspheres formed at different drug/chitosan weight ratios.
Figure 1.
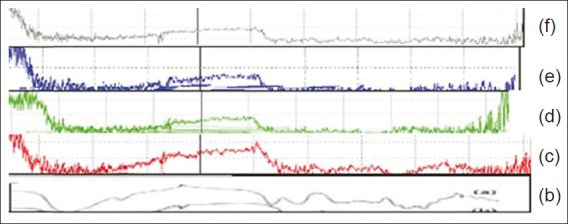
FTIR spectra of Levosalbutamol sulphate (a), chitosan (b), physical mixture (c), and 3 batches of microspheres (d-f)
Optical microscopic studies
Photomicrographs of loaded (1:1, 1.5:1 and 2:1 polymer/drug weight ratio) microspheres showed a regular shape and smooth surface; Further their size range checked by stage micrometer were in range of 5-6 μm., which is suitable for targeted area in treatment of asthma by inhalation.
Swelling studies
Accurately weighted amounts of microspheres were immersed in a little excess of PBS (pH 6.4) and kept for 24 hr and degree of swelling was calculated. Degree of swelling is proportional to ratio of polymer to drug, and it affects the property of drug release from polymer, which was shown in Table 1.
Table 1.
Swelling study

Mucoadhesion properties
Percentage of mucoadhesion was also proportional to Drug: Polymer ratio as shown in Table 2. Here batch 2 and batch 3 have similar mucoadhesion property, which was suitable for this mucoadhesive formulation.
Table 2.
Mucoadhesion study

Entrapment efficiency
Entrapment efficiency of microsphere was found to be 85%, 92% and 89.2% for B1, B2 and B3 respectively. Thus the aim was fulfilled by batch 2.
In-vitro release studies
The release data of microspheres are shown in Figure 2. The lowest drug release was obtained with Batch 3. In fact, the presence of increasing amounts of Chitosan from B1 to B3 produced a more hydrated and viscous network in the gelled microspheres thus limiting drug diffusion. Moreover, it was also influenced Levosalbutamol availability according to swelling behavior. Finally, the drug availability decreased by raising the polymer/drug weight ratio from 1:1 to 2:1. This release study follows Higuchi and Kross Meyer Pepas model.
Figure 2.
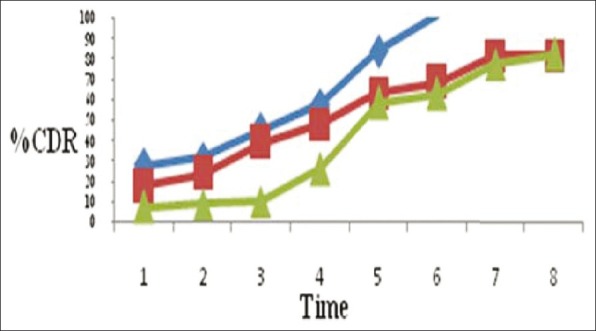
Levosalbutamol release profiles from B1 (♦), B2 (■) and B3 (▲) microspheres
Conclusion
Microspheres of Levosalbutamol prepared by above method could be given in asthma by inhalation with characteristic of sustained release action due to their appropriate particle size, swellability, and mucoadhesion characteristics.
Acknowledgement
Levosalbutamol sulphate was gifted from Marck Bioscience, Kheda, Gujrat.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Jain S. K, Chourasia M. K, Jain A. K, Jain R. K. Development and characterization of mucoadhesive microspheres bearing salbutamol for nasal delivery. Drug delivery. 2004 doi: 10.1080/10717540490280750. [DOI] [PubMed] [Google Scholar]
- 2.Corrigan Deirdre O, Healy Anne Marie, Corrigan Owen I. Preparation and release of salbutamol from chitosan and chitosan co-spray dried compacts and multiparticulates. European journal of pharmaceutics and bio pharmaceutics. 2006 doi: 10.1016/j.ejpb.2005.09.008. [DOI] [PubMed] [Google Scholar]


