Abstract
Mesoporous silica nanoparticles (MSNs) are introduced as chemically and thermally stable nanomaterials with well-defined and controllable morphology and porosity. It is shown that these particles possess external and internal surfaces that can be selectively functionalized with multiple organic and inorganic groups. Silica nano-particles were synthesized by chemical methods from tetraethylorthosilicate (TEOS), methanol (CH3OH) and deionised water in the presence of sodium hydroxide as catalyst at 80°C temperature. The nature and morphology of particles was investigated by scanning electron microscopy (SEM), N2 adsorption/desorption method using BET instrument and X-ray diffraction (XRD). Silica nanoparticles are applicable to a wide range of therapeutic entities from small molecule to peptides and proteins including hydrophobic and hydrophilic entities. Drug loading does not require chemical modification of the molecule; there are no changes in the drug structure or activity after loading and subsequent release of the drug. Thus, well suited to solve formulation problems associated with hydrophobic drugs such as peptide and protein drugs like cyclosporine A. Silica nanoparticles improved the solubility of poorly water soluble drugs and enhanced the absorption and bioavailability of these compounds.
KEY WORDS: Silica, nanoparticle, cyclosporine
Controlled drug-delivery systems (DDSs) are one of the most promising applications for human health care and represent an ever-evolving field for biomedical materials science. The field of nanotechnology in recent years has motivated researchers to develop nanostructured materials for biomedical applications. Choice of carrier is one of the major factors in controlled release formulation. The factors that need to be considered are
The carrier material should be biocompatible.
High loading/encapsulation of desired drug molecules.
Zero premature release, i.e., no leaking, of drug molecules.
Cell type or tissue specificity and site directing ability.
Controlled release of drug molecules with a proper rate of release to achieve an effective local concentration.
Characteristics of silica nanoparticles
Many research efforts have focused on (i) controlling the particle morphology and (ii) preparing the organic/inorganic hybrids through functionalization of the exterior and/or interior surfaces. The particle morphology and degree of functionalization were dictated by the concentration, molecular size, and hydrophilicity/hydrophobicity of the organoalkoxysilane precursors.
-
(i)
Controlling the particle morphology
- Tunable particle size: 50 to 300 nm
- Stable and rigid framework
- Uniform and tunable pore size: 2 and 6 nm
- High surface area and large pore volume: (> 900 m2/g) and (> 0.9 cm3/g)
- Unique porous structure
-
(ii)
Functionalization of silica nanoparticles.
Three dimensional structure of mesoporous silica nanoparticles (MSN)
MSN have an internal surface (i.e., cylindrical pores) and an external surface (i.e. exterior particle surface). This characteristic allows the selective functionalization of the internal and/or external surfaces of MSN with different moieties. The keystone in the development of silica mesoporous materials as DDSs is the modification or functionalization of the surface through organic groups. The conventional formulations of CyA (Sandimmun®) caused marked intra- and inter-individual variation in drug pharmacokinetics. Neoral®, another formulation, is a microemulsion of pre-concentrated CyA designed to provide better consistent absorption of the drug. After oral administration this compound is absorbed only incompletely and variably, leading to a relative bioavailability of less than 50%. In contrast to most peptides, it is particularly lipophilic. It is practically insoluble in water and is soluble in alcohol. These characteristics are favorable for encapsulation in particles. The nanoparticles formulation had a notably increased bioavailability compared with that of the commercial formulation.
Materials
Sodium hydroxide, Cetyl Trimethylammonium Bromide (CTAB), Tetraethyl Orthosilicate (TEOS), Ethanol, Conc. Hydrochloric Acid, Polaxamer, Sodium Dihydrogen Orthophosphate dehydrate, Cyclosporin A (USP), Acetonitrile HPLC grade, Methanol.
Synthesis of mesoporous silica nanoparticles
Method using CTAB as surfactant
The mixture of CTAB (1.5 g), 2.0 M NaOH (1.75ml) and water (120 ml) is heated to 80°C for 30 mins to reach pH 12.3. To this clear solution, add TEOS (2.335 g) is rapidly added via injection (stirring at 550 rpm), a white precipitate is observed after 3 mins of constant stirring. The reaction temperature is maintained at 80°C for 2 hours. The products are then isolated by hot filtration (dilute the sample with 300ml distilled water), washed with copious amount of water and methanol (5 ml ×3 times). An acid extraction is performed using mixture of methanol (100 ml), conc. HCl (1 ml) and previously prepared sample (1.0 g) at 60°C for 6 hours using hot plate. Resulting surfactant removed solid products are washed with methanol and water using the centrifugation and sonicator method. The washings were carried out until sample is clear of all surfactant.

Different types of mesoporous silica nanoparticles were synthesised using the varying amounts of the reagents as mentioned in the Table above and were named as CTAB 2.1, CTAB 2.2, CTAB 2.3 and CTAB 2.4. The prepared mesoporous nanoparticles were dried at 60°C in the oven and used for characterization.
Drug Loading on Mesoporous Silica Nanoparticles
Method 1
Dissolve 100 mg of the Cyclosporin A drug in ethanol (1 ml).
Add 100 mg of SNP to the clear solution of the drug and the solvent
Stir the sample and SNP mixture at 300 rpm for 24 hours.
Method 2
Dissolve 100 mg of the CYCLOSPORIN A in ethanol (1ml).
Add 100 mg of SNP to the clear solution of the drug and the solvent.
Stir the sample and SNP mixture at 300 rpm for 1 hour.
Characterization of Mesoporous Silica Nanoparticles and Drug Loaded MSN
The following are the various methods used for the characterization of mesoporous silica nanoparticles.
Particles size measurement
In these method the obtained final product before acid extraction, few drops of the final product is taken into the cuvet and then dissolved with deionised water and the cuvet filled with the deionised water was used for the particle size analysis using Malvern Master sizer 2000.
N2 adsortion/desorption method using Brunauer-Emmett-Teller (BET)
The freshly dried and finely ground sample of silica nanoparticles was taken in the BET sample tube and this sample was used for the analysis.
Equipment parameters
Make and Model: Gemini, Micromeritics
Surface area: 1 m2 to >900 m2
Sample quantity: Approximately 2.0 cm3.
The surface area of 2.3 nanoparticles was only one to comply with the theoretical criteria of silica nanoparticles. It has the largest surface area and pore size as compared to other silica nanoparticles prepared. Since the 2.3 silica nanoparticles had larger surface area, pore size and pore volume. The further characterization was carried out only on this sample.
Figure 1.
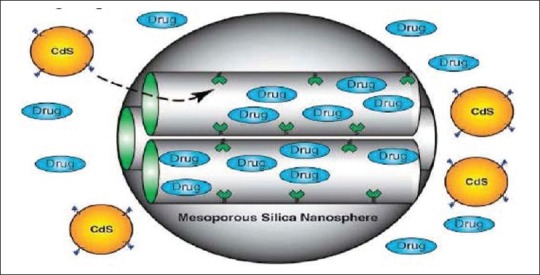
Figure 2.
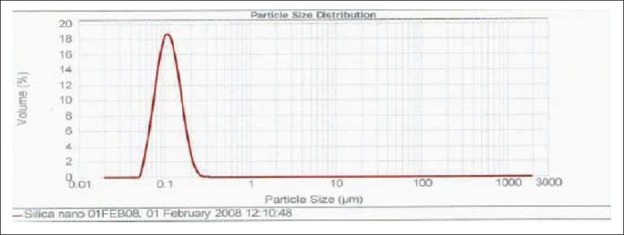
Figure 3.
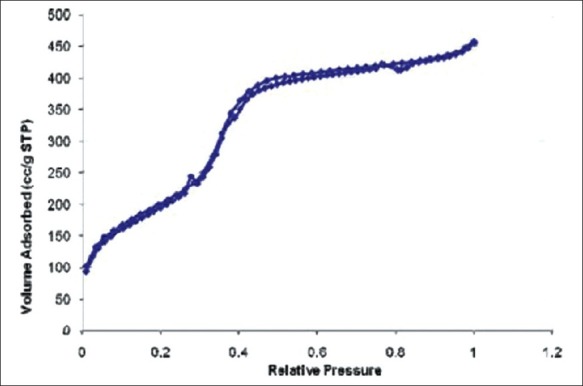
Figure 4.

Scanning electron microscopy (SEM)
From the image we can conclude that all the particles are uniform in size and have no change in the morphology after drug loading with Cyclosporin A has been carried out.
Conclusion
Mesoporous Silica Nanoparticles with larger surface area and pore volume seem to hold promise for the achievement to permit alterations in the bioavailability of drugs and improve the phamacokinetic profile of numerous drugs with biomedical purposes. The above results confirmed that the silica nanoparticles synthesized, confirmed that the amorphous nature of the Cyclosporine was not affected during the drug loading procedure as silica nanoparticles does not interact with the silica nanoparticles.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Robert A, Freitas JD., Jr what is nanomedicine? Nanomedicine: Nanotechnology, Biology, and Medicine. 2005 doi: 10.1016/j.nano.2004.11.003. 1, 2– 9h. [DOI] [PubMed] [Google Scholar]
- 2. http://www.understandingnano.com/medicine.html .


