Abstract
A new Hydrogel containing silver Sulfadiazine (SSD) was developed for enhanced burns wound healing. The hydrogel was prepared by cross-linking of PVA and Chitosan by freeze thawing method. Their gel properties, moisture retaining capacity, fluid uptake capacity, in vitro release study, in vivo burn healing effect were evaluated. Chitosan and PVA cross linking decreased gel fraction upto 70% determined the good gel properties. This cross linked hydrogel increased the Swelling ratio and Water vapour transmission rate (WVTR) which provides the sustained release of drug and moist environment for healing respectively. The hydrogel containing 7.5% of PVA, 0.75% of chitosan found to have increased gel strength, higher water vapour transmission rate and fluid uptake capacity suitable for faster healing of burns. This hydrogel also sustained the release of 1% SSD required for longer antimicrobial activity and found better in vivo burn healing capacity as compared to marketed preparation. Thus hydrogel containing 7.5% of PVA, 0.75% of chitosan and 1% SSD is a potential burns dressing with better gel properties and excellent burns healing capacity.
KEY WORDS: Silver sulfadiazine, hydrogel, freeze thaw method
Among the novel formulations to be applied on damaged skin, hydrogels have shown the superiority as they can provide a moist environment for the wound/burns and at the same time deliver the incorporated drug to the wound. Hydrogels are water-swollen polymeric materials that maintain a distinct three-dimensional structure. As dosage form, they are not greasy or oily and are water-washable. They are considered to be nontoxic and bio-compatible. Hydrogels combine the features of moist wound healing with good fluid absorbance and are transparent to allow the monitoring of healing. Hydrogels mould into the shape of wound defect will have advantages over the wound dressing patches.
Many hydrogels are prepared by physical methods such as repeated freezing and thawing, chemical methods using a cova-lent cross-linking agent including boric acid, glutaraldehyde and formaldehyde, or radiation methods using electron beam or irradiation.(PVA) hydrogels prepared with a freeze-thawing method have been studied for biomedical and pharmaceutical applications because of their non-toxicity, non-carcinogenicity and good biocompatibility. CS has anti fungal activity and proven to enhance the function of leukocytes, macrophages and fibroblasts to enhance granulation and rebuilding tissue. It is also boi-compatible and non toxic. Incorporation of SSD, first line burn/wound healing agent, further enhances the burn healing activity.
The aim of Main aim of present work is to formulate novel cross-linked hydrogel of chitosan and PVA containing Anti Microbial drug for severe burn wound. And Perform in vitro evaluation of hydrogel for gel fraction, WVTR, swelling study, drug release etc. Estimate the in vivo wound healing study of the optimized hydrogel by using rat models. Finally compare our product with marketed product.
Experimental Methods
Preparation of hydrogel
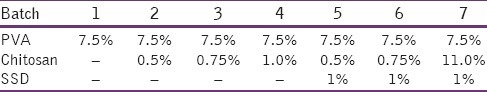
PVA/CS hydrogels were prepared by the freezing–thawing (F–T) cycle. In brief, the solutions containing different conc. of PVA and SSD were dissolved in distilled water. CS was dissolved in 0.5% acetic acid. Then they were mixed (adjust final conc. as shown in Table 1) by vortexing for 1 h and poured into Petri dishes. They were then frozen at –20°C for 18 h and then thawed at room temperature for 6 h for three consecutive cycles.
Table 1.
Compositions of PVA and CS hydrogels
Evaluation Parameters
Drug-polymer interactions by Fourier transform infrared spectrometry (FT IR), Fluid uptake ability (swelling ratio), water vapour transmission rate, gel fraction, in vitro diffusion study and in vivo burn healing study.
Results and Discussion
Drug polymer interactions by Fourier transform infrared spectrometry
To confirm the drug polymer interaction, samples were analyzed by FTIR spectrometry. Figure 1 show FTIR spectra of SSD, Chitosan, PVA and drug and polymer mixtures. The FTIR spectra shown that there is no interaction of between drug and polymers. SSD showed the characteristic absorption bands at 1,350 and 1,590 cm-1 due to –S = O bond and the stretching vibration of phenyl framework conjugated to –NH2, respectively; the stretching vibration of C–H from the phenyl framework at 3,070 cm-1 and characteristic absorption band at 2,390 and was detected for asymmetric stretching vibration of –NH2 group.
Figure 1.
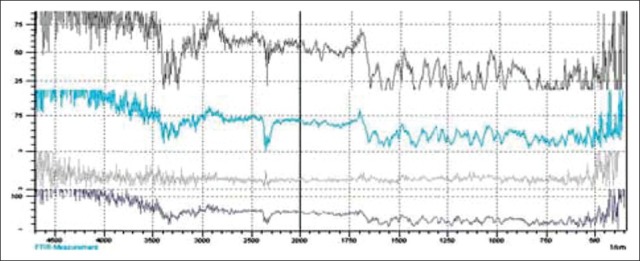
FTIR spectra of SSD, Drug+CS, Drug+PVA, Drug+PVA+CS
Fluid uptake ability (swelling ratio), water vapour transmission rate, gel fraction
The effect of different conc. of CS and PVA on gel fraction [Figure 2a] shown that as the conc. of conc. of CS increased, there were decrease in gel fractionation, determines the cross-linking interaction between PVA and CS and also indicates the mechanical strength of gels were also increased. Swelling ratio (Figure 2b) also increased as conc. of CS increased. The WVTR (Figure 2c) was highest in PVA and CS conc. of 7.5 and 0.75% respectively, around 2508 g/m2 day indicating that the hydrogel maintain a moist environment required for epithelial cell migration required during healing process.
Figure 2.
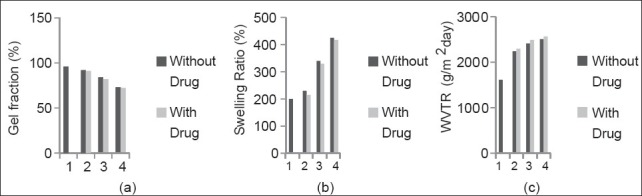
Effect of different polymer ratio onto the (a) gel fraction, (b) swelling ratio, and (c) water vapour transmission rate (WVTR)
In vitro diffusion diffusion study
Figure 3 shows that the diffusion of SSD was around 57% after 12 hour in 6th batch having CS:PVA ratio 0.75:7.5. As drug release was more sustained, it maintains anti microbial activity of SSD for longer period of time and decreases the side effects of SSD.
Figure 3.
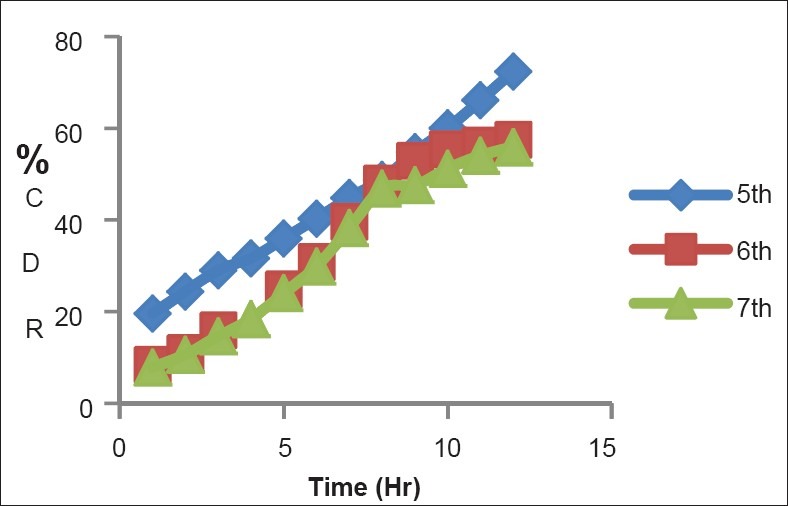
In vitro diffusion of SSD in batch 5th, 6th and 7th batch
Figure 4.
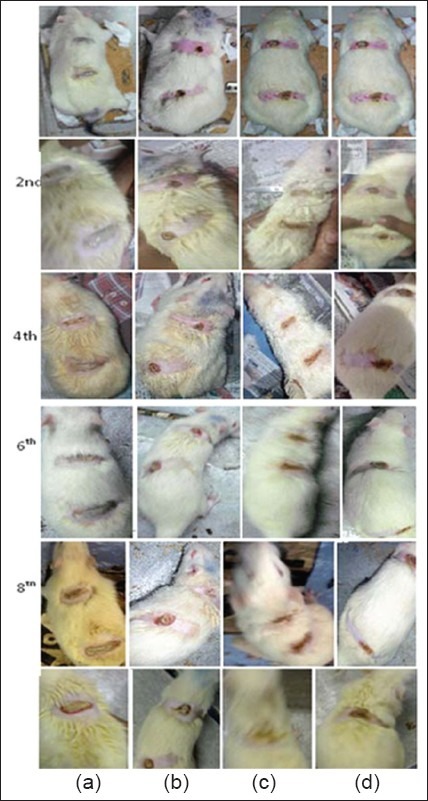
In vivo burn healing study (a) marketed preparation (b) gel without drug (c) test product (d) surgical gauze
In vivo burn healing study
As shown in Figure it was clearly observed that after 10 days animal study the burn curing rate was higher in optimized test product, it was also faster than the marketed product. And from comparing with hydrogel without drug it was observed that incorporation of drug enhances the rate of curing.
Conclusion
The Silver sulfadiazine-loaded hydrogel developed with PVA and chitosan was more swellable, flexible and elastic because of its cross-linking interaction with PVA. The hydrogel composed of 7.5% PVA, 0.75% chitosan and 1% drug significantly improved the burn healing effect compared with the gauze control, the hydrogel without drugs and the marketed product. Thus, it is a potential burn dressing with excellent forming and enhanced burn healing.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Sung J S, Yong C. Gel characterization and in vivo evaluation of minocycline wound dressing with enhanced wound healing. Int. J. Pharm. 2010;392:232–240. doi: 10.1016/j.ijpharm.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Aoyagi S, Onishi H, Machida Y. Novel chitosan wound dressing loaded with minocycline for the treatment of severe burn wounds. Int. J. Pharm. 2007;330:138–145. doi: 10.1016/j.ijpharm.2006.09.016. [DOI] [PubMed] [Google Scholar]


