Abstract
A sustain release thermo reversible in situ gel of Moxifloxacin Hydrochloride using mucoadhesive polymer was prepared. Mucoadhesive polymer was used to obtain an ophthalmic delivery system with improved mechanical and mucoadhesive properties that will provide prolong retention time for treatment of ocular diseases. Developed formulations were evaluated for drug-excipient compatibility study, pH, Clarity, Gelation temperature study, Mucoadhesion properties and in-vitro release studies. Drug-excipient compatibility study was performed by FTIR technique. The individual IR spectra of the pure drug and polymers as well as the combination spectra of the drug and polymer were taken, which indicate no interaction between Moxifloxacin and polymers when compared with infrared spectrum of pure drug as all functional group frequencies were present. The values of other parameters obtained were in acceptable range. In vitro release tests revealed that 98% drug was released from the in situ gel containing 0.5% and 1.00% HPMC in 12 hr. provides prolonged release.
KEY WORDS: Mucoadhesion, thermoreversible in situ gel, ophthalmic delivery
Ophthalmic drug delivery is one of the most interesting and challenging endeavours facing the pharmaceutical scientist. Moxifloxacin is an 8-methoxy fluoroquinolone (4th generation) having half-life nearly 12 hours. It has a broad-spectrum antibiotic activity and indicated for severe infections. The conventional liquid ophthalmic delivery systems exhibit short precorneal residence time due to rapid elimination induced by lachrymal flow, blinking, normal tear turnover, and solution drainage by gravity. Several in situ gelling systems have been developed to prolong the precorneal residence time of a drug. The sol-gel transition can be induced by a shift in pH, temperature or ion activated systems. Poloxamer (temperature sensitive 407 and 188) are used to formulate in situ hydrogel along with HPMC (K4M) as Bioadhesive polymer. In the light of above facts the present investigation is intended to formulate and evaluate the Thermo reversible insitu gel of Moxifloxacin Hydrochloride, using mucoadhesive polymer in view of increasing precorneal residence time and bioavailability of drug.
Subjects and Methods
Preparation of ophthalmic in situ gel
The gel formulation was prepared aseptically using the cold method. The drug was accurately weighed and dissolved in sterile distilled deionized water and mixed with mucoadhesive polymer solution (HPMC K4M).To this solution, required amounts of PF 407/PF 188 were added. All other excipients such as benzalkonium chloride (0.001%, m/V), sodium chloride 0.5% (m/V), were added to the mixture. The volume was adjusted with sterile distilled deionized water to achieve a 0.5% (m/V) concentration of Moxifloxacin hydrochloride. The solutions were adjusted to a pH in the range from 6.8 to 7.4 with 2 mol L–1 NaOH. The solutions were mixed well and stored at 2–8°C for 24 h.
Characterizations by Fourier transform infrared spectrometry (FT IR): The Infra-Red spectra of Moxifloxacin Hydrochloride, poloxamers, HPMC K4M and physical mixtures were obtained on Fourier Transform Infrared Spectrophotometer (Perkin Elmer – spectrum Bx, USA) in order to detect the existence of interaction between drug and polymer.
pH
pH of prepared in situ gel was measured with pH meter.
Clarity
Clarity test was done by visual inspection of each container under a good light, baffled against reflection into the eyes and viewed against reflection into the eyes and viewed against a black and white background, with the contents set in motion with a swirling action.
Gelation temperature
A transparent vial containing 10 mL of sample solution and a magnetic bar was placed in a low-temperature water bath. The temperature at which the magnetic bar completely stopped moving because of gelation was regarded as the GT. Also after tear dilution GT was measured.
Viscosity
The dynamic viscosity of the in situ gel was measured using a Brookfield viscometer (LV PRO +). The measurements was performed in triplicate, and the mean viscosity of the samples calculated. By plotting graph of shear rate vs. shear stress, non-Newtonian flow pattern was checked.
Mucoadhesion properties
Dispersions were prepared by adding 2 ml of formulation to a 20% (wt/vol) mucin dispersion in simulated tear fluid. The viscosity measurements of alone and mixture was done. Index was calculated by formula.
In-vitro release studies
The Franz diffusion cell was used for studying the in vitro release of the in situ gel. A cellulose acetate membrane (Dialysis membrane with 25 mm diameter) was adapted to the terminal portion of the cylindrical donor compartment. 1 mL in situ gel containing drug, sufficient for establishing sink conditions for the assay was placed into the donor compartment. The receptor compartment contained 15 mL of Phosphate buffer solution of pH 7.4 maintained at 37°C under mild agitation using a magnetic stirrer. At specific time intervals, aliquots of 1mL were withdrawn and immediately restored with the same volume of fresh phosphate buffer. The amount of drug released was assessed by measuring the absorbance at 289 nm using UV spectrophotometers. In order to analyse the drug release mechanism, in vitro release data was fitted into a zero-order, first order, Higuchi, Hixon-Crowell cube root law, Korsmeyer-peppas model.
Results and Discussion
To confirm the interaction of drug with Poloxamers and HPMCK4M, samples were analyzed by FT-IR. Moxifloxacin Hydrochloride showed the typical NH (1°) band at 2949 cm-1, 3530(-OH) and 1353 cm-1 (F). Combination spectra of the drug and polymer were taken, which indicate no interaction between Moxifloxacin and polymers when compared with infrared spectrum of pure drug as all functional group frequencies were present, indicates no interaction. The clarity and pH of all the formulations was within the acceptable range and hence would not cause any irritation upon administration.
Poloxamer solutions with a sufficiently high concentration were converted to gels at or above GT, which attribute to the interaction between hydrophilic PEO and hydrophobic PPO blocks. When the concentration and the temperature of the polymer are above a critical value, poloxamer molecules in the aqueous solution will self-assemble to form spherical micelles with a dehydrated PPO core surrounded by hydrated swollen PEO chains. Poloxamer analogs possess a different PEO: PPO ratio, and the different PEO: PPO ratios will lead to a different GT. Therefore, the aim of the modulation of GT can be reached by mixing various amounts of P407 and P188 in aqueous solution without affecting the gelling mechanism. Table 2 shows that the GT of the mixed poloxamer formulations was altered by PF68 concentration. HPMC K4M has also delay the GT by affecting on PEO –PPO interaction. Dilution by tears (25μl(instilled vol):7μl(tear vol)) will also contribute to GT.
Table 2.
Preliminary evaluation studies of formulations
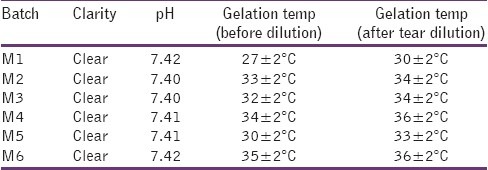
Table 1.
Composition of formulation batches
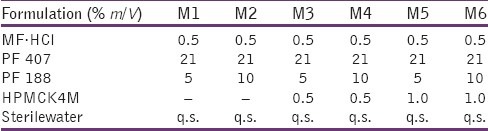
Table 3.
Kinetic profile of formulations

For thermosetting gels, the viscosity at various conditions is an important rheological parameter involved in its utilization and in vivo performance. For instance, if viscosity is too high it will lead to difficult instillation; on the contrary, if viscosity is too low it will give rise to increased drainage. As shown in Figure 2, the viscosity of poloxamer formulations was very low before STF dilution at 25°C (i.e. shown as M), which showed the character of Newtonian fluid. The gels formed at physiological conditions, and the viscosity of the formulations decreased as the shear rate increased, which showed the character of pseudo plastic fluid (i.e. shown as m).The pseudo plastic property of poloxamer formulations under physiological conditions was in favour of sustaining drainage of drugs from the conjunctival sac of the eye.
Figure 2.
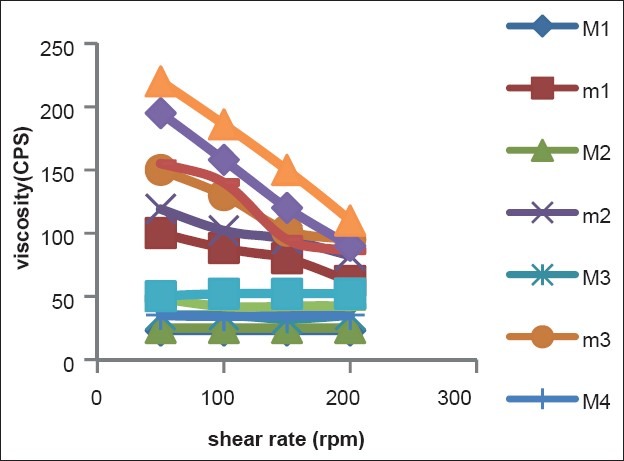
Rheograms of formulations
Figure 1.

FT-IR spectra of [a] Pure drug, [b] Drug+PF 407/188, [c] Drug+ PF 407/188+HPMCK4M
Figure 3.
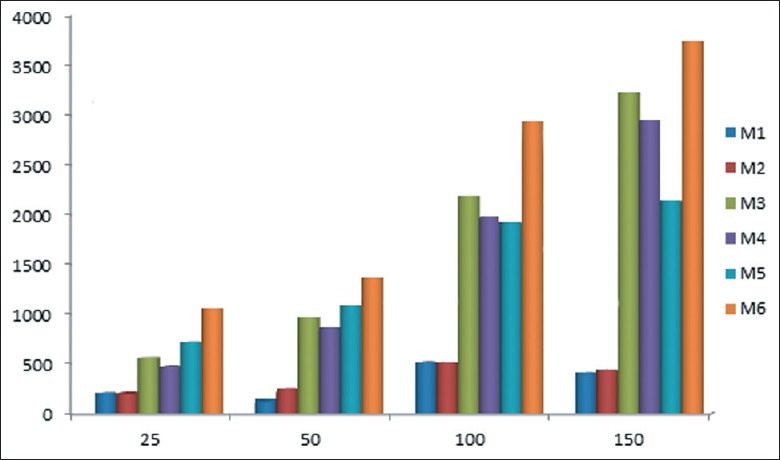
Mucoadhesion study, expressed as mucoadhesion index
Figure 4.

Assembly of diffusion study by using cellulose acetatemembrane
Mucoadhesion index was measured by 15%W/V mucin dispersion, as per following formula:
Index=D* ηmu: Where ηmu = ηtotal-ηpolymer-ηmucin
Mucoadhesion test of the batches M1 and M2 containing only Poloxamer in different concentrations was exhibited very poor mucoadhesion strength due to non-ionic nature and exhibited poor bond formation with mucin. As HPMC was added in formulation interaction was exhibited. Polymer–mucins interactions include chain interlocking, conformational changes and non-covalent bond formation. In combination of HPMC (1%) and Poloxamer batches M5 and M6 showed excellent mucoadhesion strength and higher viscosity of solutions due to HPMC was contributed for viscosity as well as mucoadhesion strength.
The viscosity of HPMC K4M was an important factor and affects the release behavior of the drug from in situ gel. The dissolution results are shown in Figure 5. Drug release up to 98% was occurred in 12 hours in case of batches M3, M4 and M6. The rate and extent of drug release was decreased with increasing the amount of HPMC K4M.
Figure 5.

In vitro drug release profile of formulations
Conclusion
The present investigation suggests when poloxamers were used in combination with HPMC desired gelation temperature, mucoadhesion, drug release profile and viscosity achieved for a sustained ophthalmic drug delivery system of Moxifloxacin hydrochloride. Kinetic model applied and suggests that all formulations follows non-fickian diffusion mechanism and the better drug release were achieved which reduce frequent instillation of eye drops.
Acknowledgement
Authors are thankful to Mr Ashok Modi and Marck Bioscience Ltd., Kheda for providing the facilities for the present work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Shastri D.H, Prajapati S.T, Patel L.D. Thermo reversible mucoadhesive ophthalmic in-situ hydrogel: Design and optimization using a combination of polymers. Acta Pharm. 2010;60:349–360. doi: 10.2478/v10007-010-0029-4. [DOI] [PubMed] [Google Scholar]
- 2.Qian Y, Wang F, Li R, Zhang Q, Xu Q. Preparation and evaluation of in situ gelling ophthalmic drug delivery system for Methazolamide. Drug Development and Industrial Pharmacy. 2010;36:1340–1347. doi: 10.3109/03639041003801893. [DOI] [PubMed] [Google Scholar]


