Abstract
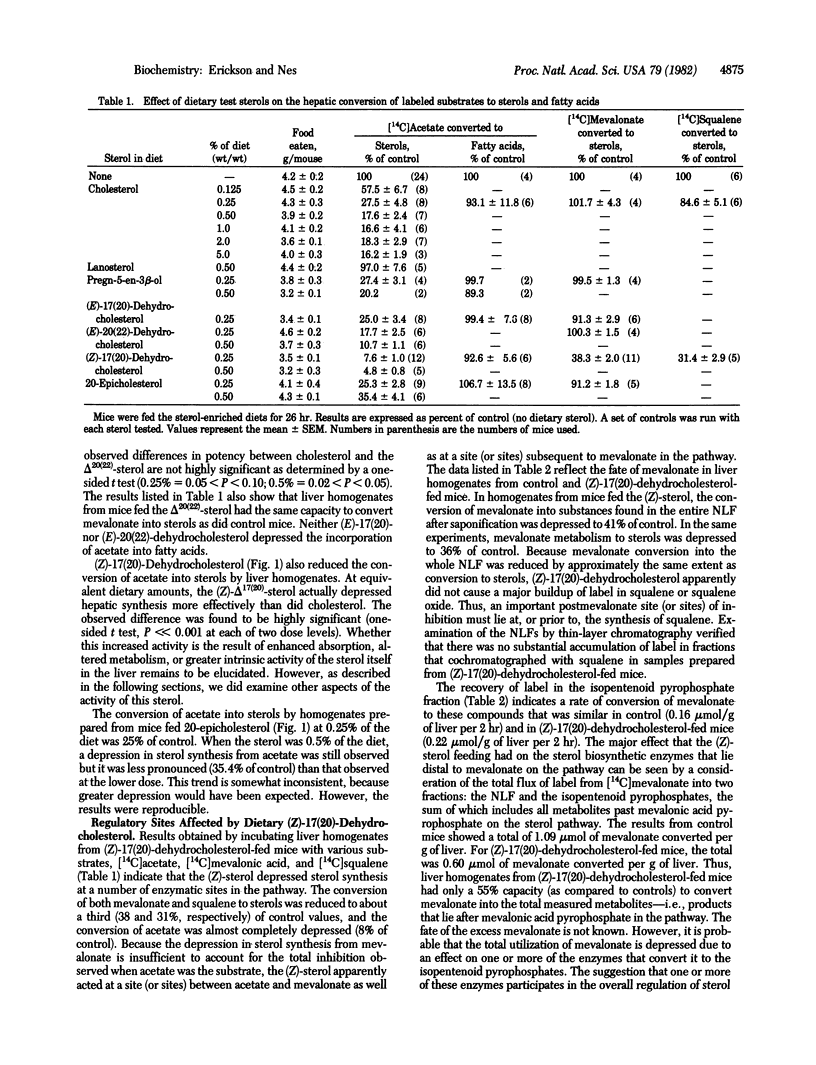
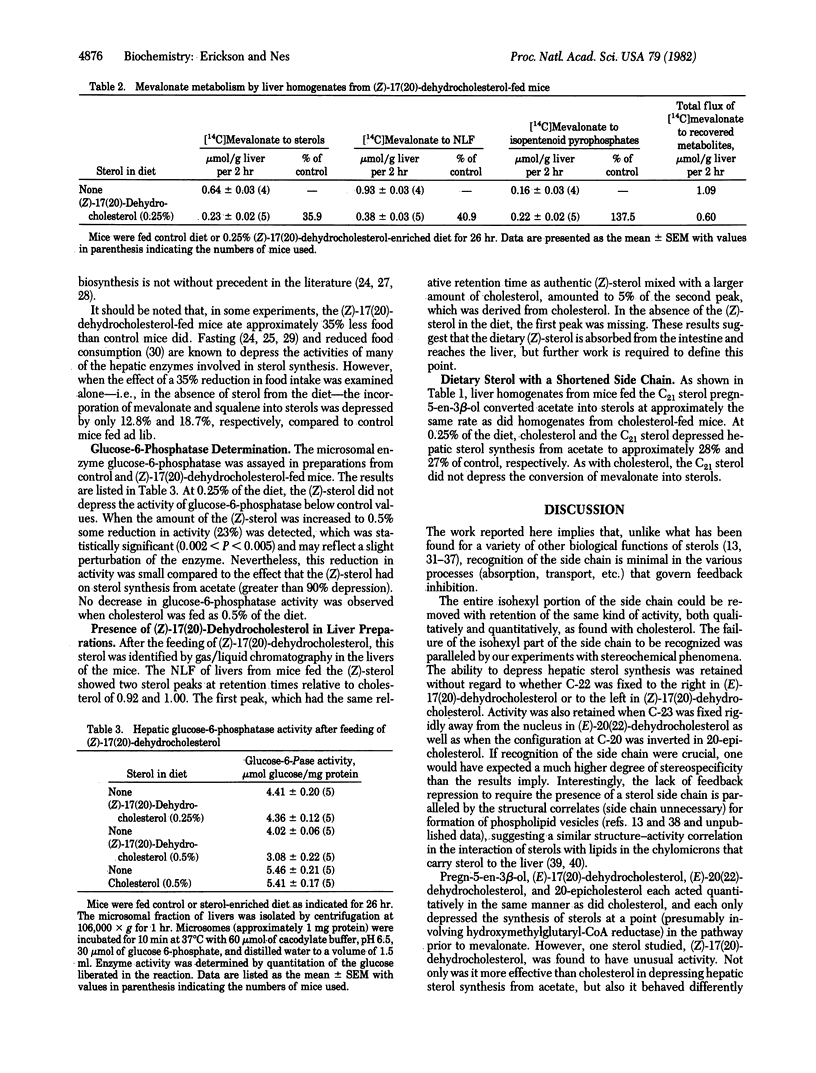
Mice were fed cholesterol or various other sterols for 26 hr, after which the amount of hepatic cholesterol synthesis was measured in a cell-free system. The following sterols were as effective as cholesterol itself in depressing the conversion of acetate into sterol: pregn-5-en-3 beta-ol, which lacks an isohexyl group on C-20; (E)-17(20)-dehydrocholesterol, in which the isohexyl group is fixed to the right; (E)-20(22)-dehydrocholesterol, in which C-23 is oriented away from the nucleus; and 20-epicholesterol. Moreover, when the isohexyl group was fixed to the left in (Z)-17(20)-dehydrocholesterol, this dietary sterol, identified in the liver, caused not only a depression in the conversion of both mevalonate and squalene into sterols. The incorporation of acetate into fatty acids was not depressed, nor did the (Z)-sterol appear to have a generalized effect on membranous enzymes, because the activity of glucose-6-phosphatase was unaffected. Thus, feedback inhibition was retained when the stereochemistry of cholesterol's side chain was drastically changed and even after the nearly complete removal of the side chain. This implies that the side chain is only minimally recognized by the mechanisms involved in feedback inhibition.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUCHER N. L., MCGARRAHAN K. The biosynthesis of cholesterol from acetate-1-C14 by cellular fractions of rat liver. J Biol Chem. 1956 Sep;222(1):1–15. [PubMed] [Google Scholar]
- BUCHER N. L., McGARRAHAN K., GOULD E., LOUD A. V. Cholesterol biosynthesis in preparations of liver from normal, fasting, x-irradiated, cholesterol-fed, triton, or delta 4-cholesten-3-one-treated rats. J Biol Chem. 1959 Feb;234(2):262–267. [PubMed] [Google Scholar]
- Boyd G. S., Brown M. J., Hattersley N. G., Suckling K. E. Studies on the specificity of the rat liver microsomal cholesterol 7alpha-hydroxylase. Biochim Biophys Acta. 1974 Jan 23;337(1):132–135. doi: 10.1016/0005-2760(74)90047-2. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res. 1980 Jul;21(5):505–517. [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Receptor-mediated control of cholesterol metabolism. Science. 1976 Jan 16;191(4223):150–154. doi: 10.1126/science.174194. [DOI] [PubMed] [Google Scholar]
- Chang T. Y., Limanek J. S. Regulation of cytosolic acetoacetyl coenzyme A thiolase, 3-hydroxy-3-methylglutaryl coenzyme A synthase, 3-hydroxy-3-methylglutaryl coenzyme A reductase, and mevalonate kinase by low density lipoprotein and by 25-hydroxycholesterol in Chinese hamster ovary cells. J Biol Chem. 1980 Aug 25;255(16):7787–7795. [PubMed] [Google Scholar]
- Duax W. L., Griffin J. F., Rohrer D. C., Weeks C. M. Conformational analysis of sterols: comparison of X-ray crystallographic observations with data from other sources. Lipids. 1980 Sep;15(9):783–792. doi: 10.1007/BF02534032. [DOI] [PubMed] [Google Scholar]
- Erickson S. K., Cooper A. D., Matsui S. M., Gould R. G. 7-Ketocholesterol. Its effects on hepatic cholesterogenesis and its hepatic metabolism in vivo and in vitro. J Biol Chem. 1977 Aug 10;252(15):5186–5193. [PubMed] [Google Scholar]
- Erickson S. K., Matsui S. M., Shrewsbury M. A., Cooper A. D., Gould R. G. Effects of 25-hydroxycholesterol on rat hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in vivo, in perfused liver, and in hepatocytes. J Biol Chem. 1978 Jun 25;253(12):4159–4164. [PubMed] [Google Scholar]
- GOODMAN D. S., POPJAK G. Studies on the biosynthesis of cholesterol. XII. Synthesis of allyl pyrophosphates from mevalonate and their conversion into squalene with liver enzymes. J Lipid Res. 1960 Jul;1:286–300. [PubMed] [Google Scholar]
- Gould R. G., Swyryd E. A. Sites of control of hepatic cholesterol biosynthesis. J Lipid Res. 1966 Sep;7(5):698–707. [PubMed] [Google Scholar]
- Holick M. F., Garabedian M., Schnoes H. K., DeLuca H. F. Relationship of 25-hydroxyvitamin D3 side chain structure to biological activity. J Biol Chem. 1975 Jan 10;250(1):226–230. [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W., Heiniger H. J. Biological activity of some oxygenated sterols. Science. 1978 Aug 11;201(4355):498–501. doi: 10.1126/science.663671. [DOI] [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W. Inhibition of sterol synthesis in cultured mouse cells by cholesterol derivatives oxygenated in the side chain. J Biol Chem. 1974 Oct 10;249(19):6057–6061. [PubMed] [Google Scholar]
- Kandutsch A. A., Heiniger H. J., Chen H. W. Effects of 25-hydroxycholesterol and 7-ketocholesterol, inhibitors of sterol synthesis, administered orally to mice. Biochim Biophys Acta. 1977 Feb 23;486(2):260–272. doi: 10.1016/0005-2760(77)90022-4. [DOI] [PubMed] [Google Scholar]
- Kandutsch A. A., Packie R. M. Comparison of the effects of some C27-, C21-, and C19-steroids upon hepatic sterol synthesis and hydroxymethylglutaryl-CoA reductase activity. Arch Biochem Biophys. 1970 Sep;140(1):122–130. doi: 10.1016/0003-9861(70)90016-0. [DOI] [PubMed] [Google Scholar]
- Karle J. Determination of molecular formula and stereoconfiguration of unique steroids by X-ray diffraction analysis. Lipids. 1980 Sep;15(9):793–797. doi: 10.1007/BF02534033. [DOI] [PubMed] [Google Scholar]
- LANGDON R. G., BLOCH K. The effect of some dietary additions on the synthesis of cholesterol from acetate in vitro. J Biol Chem. 1953 May;202(1):77–81. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nes W. D., Patterson G. W., Bean G. A. Effect of Steric and Nuclear Changes in Steroids and Triterpenoids on Sexual Reproduction in Phytophthora cactorum. Plant Physiol. 1980 Nov;66(5):1008–1011. doi: 10.1104/pp.66.5.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nes W. R., Adler J. H., Billheimer J. T., Erickson K. A., Joseph J. M., Landrey J. R., Marcaccio-Joseph R., Ritter K. S., Conner R. L. A comparison of the biological properties of androst-5-en-3 beta-ol, a series of (20R)-n-alkylpregn-5-en-3 beta-ols and 21-isopentylcholesterol with those of cholesterol. Lipids. 1982 Mar;17(3):257–262. doi: 10.1007/BF02535113. [DOI] [PubMed] [Google Scholar]
- Nes W. R., Joseph J. M., Landrey J. R., Behzadan S., Conner R. L. Steric effects at C-20 and C-24 on the metabolism of sterols by Tetrahymena pyriformis. J Lipid Res. 1981 Jul;22(5):770–777. [PubMed] [Google Scholar]
- Nes W. R., Joseph J. M., Landrey J. R., Conner R. L. The effects of branching, oxygen, and chain length in the side chain of sterols on their metabolism by Tetrahymena pyriformis. J Biol Chem. 1980 Dec 25;255(24):11815–11821. [PubMed] [Google Scholar]
- Nes W. R., Joseph J. M., Landrey J. R., Conner R. L. The steric requirements for the metabolism of sterols by Tetrahymena pyriformis. J Biol Chem. 1978 Apr 10;253(7):2361–2367. [PubMed] [Google Scholar]
- Nes W. R., Sekula B. C., Nes W. D., Adler J. H. The functional importance of structural features of ergosterol in yeast. J Biol Chem. 1978 Sep 10;253(17):6218–6225. [PubMed] [Google Scholar]
- Nes W. R., Varkey T. E. (Z)-17(20)-dehydrocholesterol. A new sterol with C-21 and C-22 spatially fixed. J Org Chem. 1976 Oct 15;41(21):3429–3433. doi: 10.1021/jo00883a023. [DOI] [PubMed] [Google Scholar]
- Nes W. R., Varkey T. E., Krevitz K. The stereochemistry of sterols at C-20 and its biosynthetic implications. J Am Chem Soc. 1977 Jan 5;99(1):260–262. doi: 10.1021/ja00443a054. [DOI] [PubMed] [Google Scholar]
- Nes W. R., Varkey T. E., Krevitz K. The stereochemistry of sterols at C-20 and its biosynthetic implications. J Am Chem Soc. 1977 Jan 5;99(1):260–262. doi: 10.1021/ja00443a054. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Johnson R. L., Corradino R., Okamura W. H. Structure-function studies of the side chain of 25-hydroxyvitamin D3. J Biol Chem. 1979 Nov 25;254(22):11445–11449. [PubMed] [Google Scholar]
- Siperstein M. D., Fagan V. M. Feedback control of mevalonate synthesis by dietary cholesterol. J Biol Chem. 1966 Feb 10;241(3):602–609. [PubMed] [Google Scholar]
- Slakey L. L., Craig M. C., Beytia E., Briedis A., Feldbruegge D. H., Dugan R. E., Qureshi A. A., Subbarayan C., Porter J. W. The effects of fasting, refeeding, and time of day on the levels of enzymes effecting the conversion of -hydroxy- -methylglutaryl-coenzyme A to squalene. J Biol Chem. 1972 May 25;247(10):3014–3022. [PubMed] [Google Scholar]
- Speck S. H., Ferguson-Miller S., Osheroff N., Margoliash E. Definition of cytochrome c binding domains by chemical modification: kinetics of reaction with beef mitochondrial reductase and functional organization of the respiratory chain. Proc Natl Acad Sci U S A. 1979 Jan;76(1):155–159. doi: 10.1073/pnas.76.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suckling K. E., Blair H. A., Boyd G. S., Craig I. F., Malcolm B. R. The importance of the phospholipid bilayer and the length of the cholesterol molecule in membrane structure. Biochim Biophys Acta. 1979 Feb 20;551(1):10–21. doi: 10.1016/0005-2736(79)90349-3. [DOI] [PubMed] [Google Scholar]
- TOMKINS G. M., SHEPPARD H., CHAIKOFF I. L. Cholesterol synthesis by liver. III. Its regulation by ingested cholesterol. J Biol Chem. 1953 Mar;201(1):137–141. [PubMed] [Google Scholar]
- Tavani D. M., Nes W. R., Billheimer J. T. The sterol substrate specificity of acyl CoA: :cholesterol acyltransferase from rat liver. J Lipid Res. 1982 Jul;23(5):774–781. [PubMed] [Google Scholar]


