Abstract
Periodontitis is an inflammatory disease of the supporting tissues of the teeth, caused by a group of specific microorganisms. Aggressive forms of periodontitis can be localized or generalized. The concept that localized problem sites may be treated by local drug delivery appears attractive as the antimicrobial agent is delivered within periodontal pockets and the therapy is targeted on specific pathogenic microorganisms. Periodontitis can result in bone resorption creating bony defects, which may cause tooth loss. Various drugs have been studied using local delivery to improve the periodontal health and to achieve periodontal regeneration. Local delivery of antimicrobial agents using controlled release systems should be considered as adjunctive to mechanical debridement for the treatment of localized forms of periodontal destruction. Pharmacological agents offer great promise in this direction. Simvastatin, used for the treatment of hypercholesterolemia, is a universally accepted and relatively inexpensive drug. Local application of simvastatin has been shown to stimulate bone formation in rodents both in vitro and in vivo and in human periodontal ligament cells in vitro. This article reviews the effects of simvastatin as a local delivery and examines its potential role in periodontal regenerative therapy.
KEY WORDS: Local drug delivery simvastatin, periodontal regeneration, periodontitis
Recent development of science and technology has revolutionized the basic outlook and approach to the problems of periodontal disease. It is a well-established fact today that the many variants of periodontal disease are essentially infections to the periodontal structures, with differing microorganisms predominating in the different presentations of the disease. Chronic inflammatory periodontal disease is highly prevalent, especially in late middle age, when cardiovascular disease is also common. In periodontitis, the production of proinflammatory cytokines and tissue-degradative enzymes is initiated and advanced by oral bacterial infection, ultimately resulting in the destruction of periodontal tissue. Earlier, it was assumed that periodontal problems were invariably progressive and the morbid effects increase with passage of time. A thorough understanding of the etiopathogenesis of periodontal disease has provided the clinicians and researchers with a number of diagnostic tools and techniques, which has widened the treatment options.[1]
The clinical signs of periodontitis are changes in the morphology of gingival tissues, bleeding upon probing, as well as periodontal pocket formation. This pocket provides an ideal environment for the growth and proliferation of anaerobic pathogenic bacteria.[2] The microorganisms colonizing the subgingival area represent the principal etiological factor in the development of the inflammation and tissue destruction occurring adjacent to bone. Periodontitis leads to bone resorption, creating bony defects that may cause tooth loss.[2] The microflora found in periodontitis is complex and composed mainly of Gram-negative anaerobic bacteria.[3] Suspected periodontal pathogens have been shown to produce a large number of biological molecules that may act directly on host tissue and destroy its integrity. The aim of current periodontal therapy is to remove the bacterial deposits from the tooth surface and to shift the pathogenic microbiota to one compatible with periodontal health. Therapeutic approaches include mechanical scaling and root planing (SRP) and, in some cases, surgery.
Systemically applied antimicrobials have been advocated for the treatment of severe forms of periodontitis. However, in the early 1970s, concern emerged with respect to systemic antibiotic therapy for chronic infections such as periodontal disease. The drawbacks would be markedly reduced if antimicrobial agents applied locally could be used. Topical administration of antibacterial agents in the form of mouthwashes has been shown to be effective in controlling supragingival plaque (Kornman, 1986). However, their access to the periodontal pocket and the subgingival flora is limited (Flotra, 1973; Pitcher et al., 1980) and therefore ineffective in controlling disease progression. Local delivery of chemotherapeutic agents into the pockets via a syringe or irrigating device has been shown to have an effect on the subgingival flora (Addy et al. 1984). A number of studies have concentrated on the effects of locally administered simvastatin (SMV) on bone formation. The local tissue concentration of a drug can be enhanced by incorporating the active agent into controlled release delivery systems to be placed directly in the periodontal pocket or the defect area. This article reviews the effects of SMV and examines its potential role in periodontal regenerative therapy when applied locally.
Principle of Local Delivery
A local delivery system can be considered:
as an adjunct in periodontal therapy, that is, it can be considered to be an extension of SRP,
to be an add-on supportive therapy in conjunction with systemic antibiotic therapy, or
a key element of the complex form of therapy for the management of periodontal disease.[1]
The local drug delivery is used as an extension of SRP. The rationale for the use of the local drug under such situations is to eliminate any residual infective/inflammatory component still harboring in the periodontal apparatus. Since SRP is a general purpose task carried out in minimal timing with sophisticated instruments, the application of local drugs should also be as simple and fast. In such situations, the ideal form of local drug is the gel form (Ainamo et al. 1992) which will allow for very fast, generalized application in all afflicted areas. If local drugs are used as an add-on to systemic drug therapy for severe infective lesions of the periodontium, the most important criterion is the prolonged availability of the drug in sufficient minimum inhibitory concentrations over a number of days.
Delivery Systems
The most critical aspect of a local drug delivery system is the method by which the active ingredient is made available at the site of action.
Cupraphane Cellulose Hollow Fiber Systems
Dr. Max Goodson, in early 1980, loaded these fibers with systemic tetracycline and placed them in deep lesions. The downside was the necessity to remove the hollow fibers and the drug slipping out of the pocket much before the tetracycline had played its role (Goodson et al. 1991).
Mucus Adhesive Gels
Gels are usually supplied in thin syringes with blunt-ended needles. The drawback is that the needles are very thick and the amount of pressure required to force the gel out of the needle makes the entire placement of the syringe/needle very unstable (Ainamo et al. 1992).
Gel delivery Syringe
The system consists of a cartridge which has to be loaded with the gel, and then controlled amounts of the gel can be expressed out through a blunt-ended endodontic 26/28 gauge needle tip (Steenberghe et al. 1993).
Synthetic Polymer Chips
One system is available in which the active ingredient is delivered via a non-resorbable polymer-based semi-flexible chip. The polymer, by itself, is inert and will lie in the pocket for a certain period and then chemically disintegrate (Medlicott et al.,1992).
Collagen Fiber Vehicle (Biodegradable)
This is a unique patented delivery system in which biodegradable collagen is used as a vehicle. The collagen is impregnated with the active agent, which is released over a period of 7–10 days (Garrett et al. 2006).
Drugs Used for Local Drug Delivery
Different drugs used for local delivery are tetracyclines, including doxycycline (Dunn et al. 1983; Goodson et al. 1983) and minocycline (Okuda et al. 1994), metronidazole (Hitzig et al. 1994), and chlorhexidine (Addy et al. 1984). Tetracyclines (Goodson et al. 1991) are bacteriostatic for many pathogens at concentrations found in the gingival crevicular fluid after systemic administration (3–6 μg/ml).
Simvastatin Used in Local Drug Delivery
Statins like SMV, lovastatin, and pravastatin are specific competitive inhibitors of 3-hydroxy-2-methyl-glutaryl coenzyme A (HMG-CoA) reductase.[3–5] These agents are widely used to lower cholesterol, and they provide an important and effective approach for the treatment of hyperlipidemia and arteriosclerosis. Statins also seem to modulate bone formation by increasing the expression of bone morphogenetic protein-2, inflammation, and angiogenesis,[6] thus providing a new direction in the field of periodontal therapy. Various animal studies[7–9] have shown that SMV assists in bone regeneration as well as the anti-inflammatory effect when delivered or applied locally. Sakoda et al.[10] measured the effect of SMV on interleukin (IL)-6 and -8 production in a cultured human epithelial cell line (KB cells) in response to IL-1. SMV decreased the production of IL-6 and -8, an effect that was reversed by adding mevalonate or geranylgeranyl pyrophosphate, but not farnesyl pyrophosphate. SMV reduced nuclear factor-kappa B and activator protein 1 promoter activity in KB cells, indicating an anti-inflammatory effect for SMV on human oral epithelial cells, apparently involving Rac1 GTPase (a hydrolase enzyme that can bind and hydrolyze guanosine triphosphate) inhibition. At a low concentration, SMV exhibits a positive effect on the proliferation and osteoblastic differentiation of human PDL cells, and these effects may be caused by inhibition of the mevalonate pathway. SMV is reported to stimulate vascular endothelial growth factor (VEGF) release in a dose-dependent manner and the authors suggested that statins may promote osteoblast differentiation and bone nodule formation by stimulating VEGF expression in bone tissue.[7]
Periodontal therapy necessitates a focused effect in specific defects, suggesting the importance of local application of this drug. A number of studies have concentrated on the effects of locally administered SMV on bone formation (Jeon et al. 2008; Morris et al. 2008; Vaziri et al. 2007). It has also been observed that application of this agent to a culture of human periodontal ligament cells enhances their proliferation and metabolism (Yazawa et al., 2005). Therefore, SMV could play a significant role as a therapeutic agent in the treatment of periodontal disease [Figure 1].
Figure 1.
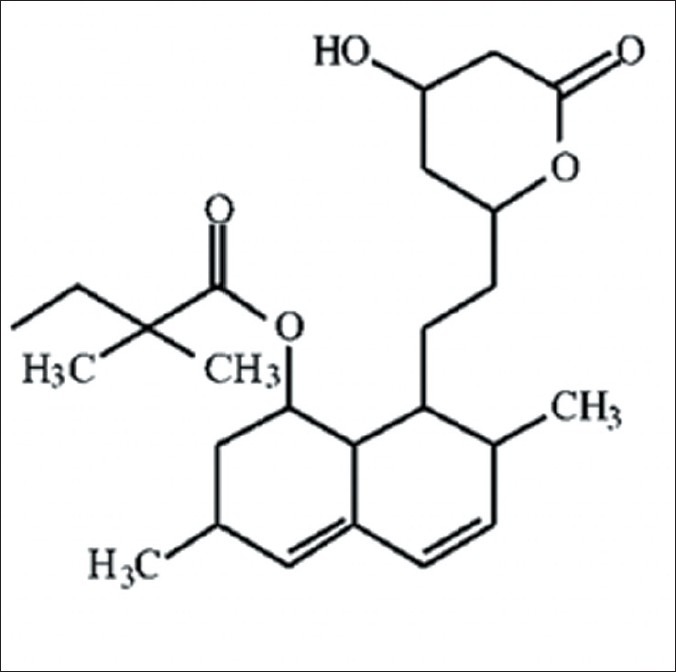
Molecular structure of simvastatin
Findings of Animal Model Studies
Mundy et al. (1999) tested the effects of more than 30,000 compounds on bone formation. They found that the addition of statins, including SMV, to neonatal murine calvarial bone in organ culture increased new bone formation by two- to threefold. A number of other workers have reported increased bone formation after local and systemic application of SMV in various animal models (Jeon et al. 2008; Morris et al. 2008; Vaziri et al. 2007; Skoglund et al. 2002; Thylin et al. 2002; Ayukawa et al. 2004, Stein et al. 2005; Skoglund and Aspenberg, 2007; Seto et al. 2008; Lee et al. 2008). The findings of these studies are encouraging from a periodontal perspective because they demonstrate a direct effect of locally applied SMV on bone formation. Bradely et al. showed that SMV stimulates BMP-2 and nitric oxide formation and regional bone formation in rat mandible models. Ozec et al (2007) examined the effect of local SMV application on 3-mm bone defects in rat mandible.
Radiologic assessment of newly formed bone by peripheral quantitative computed tomography showed significantly increased density in the experimental group. Topical dose is reported to affect a localized area of bone, whether in a 70-kg human or in a 0.3-kg rat. It was reported that even the injection of 1.5 mg/kg/week compares favorably with the 7 mg/kg/week in human oral regimens.
Effects on Bone Metabolism
Inhibition of bone resorption: Inhibition of the enzyme HMG-CoA reductase and the subsequent blockade of the mevalonate pathway (Fisher et al. 1999).
Compounds called isoprenoids (Goldstein and Brown, 1990) are primarily responsible for the prenylation of GTP-binding proteins and are involved in cytoskeletal function and vesicular trafficking. Thus, interference with the generation of isoprenoids leads to disruption of vesicular fusion and ruffled border formation of osteoclasts, which are essential for their bone resorbing activity. As a result, osteoclast inactivation occurs and bone resorption is inhibited (Fisher et al. 1999).
Local stimulation of BMP-2, a major bone growth regulatory factor, can lead to new bone formation (Takuwa et al., 1991).
SMV was also found to induce this promoter activity and appeared to be more potent than compactin (Sugiyama et al., 2000).
SMV, atorvastatin, and cerivastatin markedly enhance gene expression for VEGF in MC3T3-E1 cells (preosteoblastic murine cells) (Maeda et al., 2003).
Antioxidant and Anti-inflammatory Properties
SMV has been shown to inhibit the ability of macrophages to oxidize low-density lipoproteins (LDL) (Giroux et al., 1993).
Statins reduce the plasma levels of inflammatory markers like C-reactive protein (CRP; Davignon and Laaksonen, 1999).
Statin-mediated decrease in CRP concentrations could be due to inhibition of IL-6 in the vascular tissues (Ikeda et al. 1999).
Thus, statins, including SMV, are believed to have biologically significant antioxidant and anti-inflammatory effects, which could prove beneficial in the treatment of periodontitis.
Effects on the Periodontium
Cells derived from the periodontal ligament are believed to play an important role in the healing of alveolar bone. In vitro studies have demonstrated that they exhibit osteoblast-like properties (Arceo et al. 1991) and are responsible not only for osteogenesis and osteoclasis, but also for fibrogenesis and fibroclasis, and cementogenesis and cementoclasis (Melcher, 1976).
Yazawa et al. (2005) carried out an in vitro study using periodontal ligament cells obtained from human teeth. It was observed that SMV enhanced cell proliferation and metabolism dose dependently after 24 h. It also promoted cell proliferation significantly. The maximum effect was seen at SMV concentrations of 10-8 and 10-7 M.
After 7 days, alkaline phosphatase activity was promoted dose dependently and the maximum effect was seen at a concentration of 10-8 M.
Pradeep and Thorat (2010)[11] recently reported a greater decrease in gingival index and probing depth at sites treated with SRP and locally delivered SMV as compared to SRP plus placebo in human subjects with chronic periodontitis. In addition, more clinical attachment level gain as well as significant intrabony defect fill was seen in the SMV treated individuals.
Carriers Used
The successful use of SMV to promote bone formation in vivo depends on the local concentration and there have been continuous efforts to find an appropriate delivery system.[8] There are a number of advantages to an appropriate carrier; including localization and retention of the molecule to the site of application, thus reducing the loading dose and providing a matrix for mesenchymal cell infiltration and a substrate for cell growth and differentiation. The carrier may also help to define the shape of resulting new bone and the optimal carrier has a degradation rate that does not inhibit bone growth and prevent fibrous tissue formation or fibrous encapsulation of the carrier. There have been many studies demonstrating the osteopromotive effect achieved by the local application of the drug with different carriers in various animal models.
Gelatin sponge is biocompatible, bioresorbable, and adapts easily to the shape of defects because of its sponge-like form.
Polylactic acid/polyglycolic acid copolymer carriers with 1 mg of SMV were implanted into extraction sockets of mandibular incisors and local application of SMV is reported to preserve the residual alveolar bone effectively by promoting bone formation in the extraction socket.[9]
Critical-sized bone defects in rat calvaria were treated with calcium sulfate or with a combination of 1 mg SMV and calcium sulfate. It was reported that the combination of SMV and calcium sulfate stimulated bone regeneration.[9] Immobilization of SMV onto titanium implants is suggested to promote osteogenesis in the bone tissue surrounding the implants through its topical application.
Methylcellulose is generally regarded as a non-toxic, non-allergic, and non-irritating material and is used as a sustained release vehicle for therapeutic drugs.
Applications in periodontal therapy
Periodontitis is characterized by an inflammatory breakdown of the tooth supporting structures. Periodontal therapy aims at arresting this breakdown and restoring periodontal tissues to their original structure and function. SMV has been shown to inhibit bone resorption. However, this effect appears minor in comparison to its anabolic action on new bone formation and osteoblast maturation (Mundy et al., 1999). It also possesses anti-inflammatory and antioxidant properties (Davignon and Laaksonen, 1999). It could, therefore, have a potential role in regenerative therapy. It is administered in the prodrug form, which is much more lipophilic than the active beta-hydroxyacid form. Because of this property, the SMV molecule can effectively cross cellular membrane barriers by passive diffusion (Garrett et al. 2001). This also implies that it can be incorporated into hydrophobic delivery vehicles for local sustained release to achieve bone formation in periodontal defects. Additionally, solutions of SMV in optimal concentrations (Yazawa et al. 2005) could be combined with bone grafts to enhance their regenerative potential. The low cost and impressive long-term safety profile (Guthrie, 2006) of this compound make it a suitable agent in periodontal therapy.
Conclusion
SMV, a competitive inhibitor of the enzyme HMG-CoA reductase, is a widely used cholesterol-lowering drug. It has been found to have a number of pleiotropic effects. The findings of studies involving SMV have been encouraging and its effects on bone metabolism favor its use in the treatment of periodontal defects. As a monotherapy, local drug delivery systems incorporating a variety of drugs can improve periodontal health. SMV, as a local drug delivery, has shown to have several advantages. The antioxidant and anti-inflammatory properties of SMV could further facilitate healing of periodontal intrabony defects. However, long-term clinical studies in human subjects are required to evaluate the potential benefits of SMV in periodontal regenerative therapy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Kornman KS. Controlled release local delivery- Antimicrobials in Periodontics. Prospects for the future. J Periodontol. 1993;64:782–91. doi: 10.1902/jop.1993.64.8s.782. [DOI] [PubMed] [Google Scholar]
- 2.Goodson JM, Cugini MA, Kent RL, Armitage GC, Cobb CM, Fine D, et al. Multicenter evaluation of tetracycline fiber therapy: I. Experimental design, methods, and baseline data. J Periodont Res. 1991a;26:361–70. doi: 10.1111/j.1600-0765.1991.tb02075.x. [DOI] [PubMed] [Google Scholar]
- 3.Goodson JM. Antimicrobial strategies for treatment of periodontal diseases. Periodontology. 1994;2005:142–68. doi: 10.1111/j.1600-0757.1994.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 4.Hitzig C, Charbit Y, Bitton C, Fosse T, Teboul M, Hannoun L, et al. Topical metronidazole as an adjunct to subgingival debridement in the treatment of chronic periodontitis. Clin Perio. 1994;21:146–51. doi: 10.1111/j.1600-051x.1994.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 5.Okuda K, Wolff L, Oliver R, Osborn J, Stoltenberg J, Bereuter J, et al. Minocycline slow release formulation effect on subgingival bacteria. J Periodontol. 1992;63:73–9. doi: 10.1902/jop.1992.63.2.73. [DOI] [PubMed] [Google Scholar]
- 6.Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–9. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 7.Henwood JM, Heel RC. Lovastatin. A preliminary review of its pharmacodynamic properties and therapeutic use in hyperlipidemia. Drugs. 1988;36:429–54. doi: 10.2165/00003495-198836040-00003. [DOI] [PubMed] [Google Scholar]
- 8.Kishida Y, Naito A, Iwado S, Terahara A, Tsujita Y. Research and development of pravastatin. Yakugaku Zasshi. 1991;111:469–87. doi: 10.1248/yakushi1947.111.9_469. [DOI] [PubMed] [Google Scholar]
- 9.Todd PA, Goa KL. Simvastatin. A review of its pharmacological properties and therapeutic potential in hypercholesterolemia. Drugs. 1990;40:583–607. doi: 10.2165/00003495-199040040-00007. [DOI] [PubMed] [Google Scholar]
- 10.Sakoda K, Yamamoto M, Negishi Y, Liao JK, Node K, Izumi Y. Simvastatin decreases IL-6 and IL-8 production in epithelial cells. J Dent Res. 2006;85:520–3. doi: 10.1177/154405910608500608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pradeep AR, Thorat MS. Clinical Effect of Subgingivally Delivered Simvastatin in the Treatmen of Patients With Chronic Periodontitis: A Randomized Clinical Trial. J Periodontol. 2010;81:214–22. doi: 10.1902/jop.2009.090429. [DOI] [PubMed] [Google Scholar]


