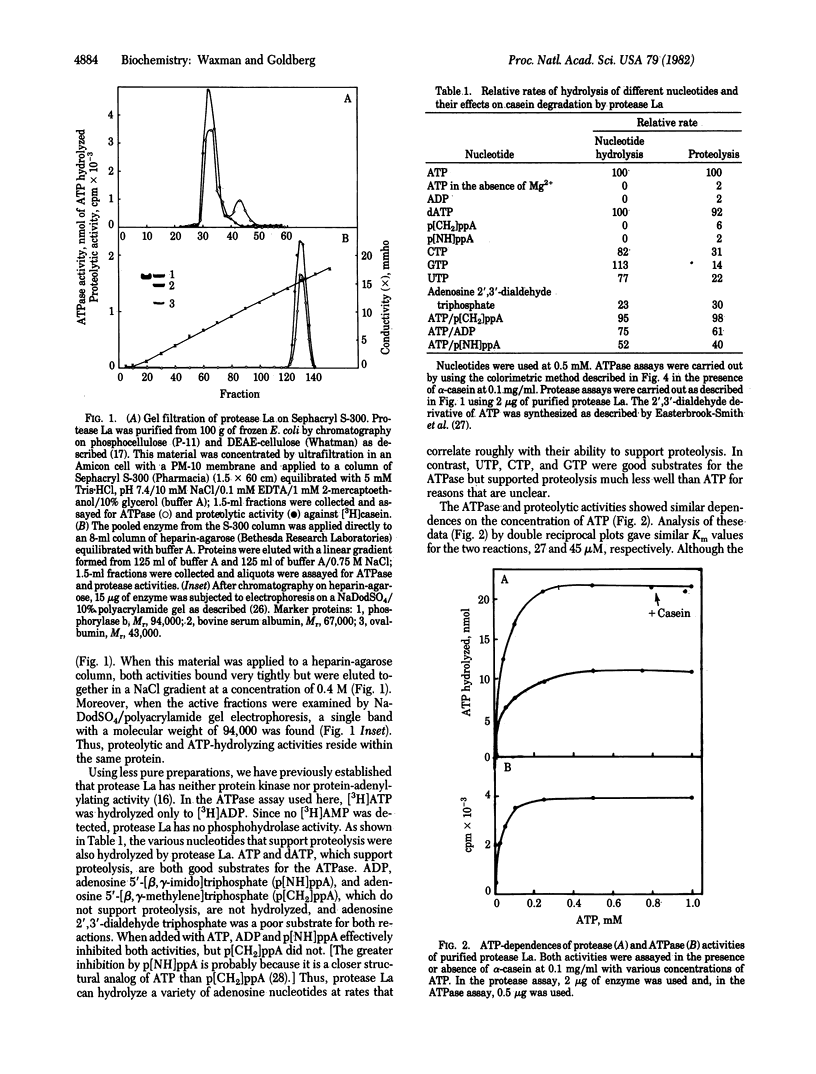
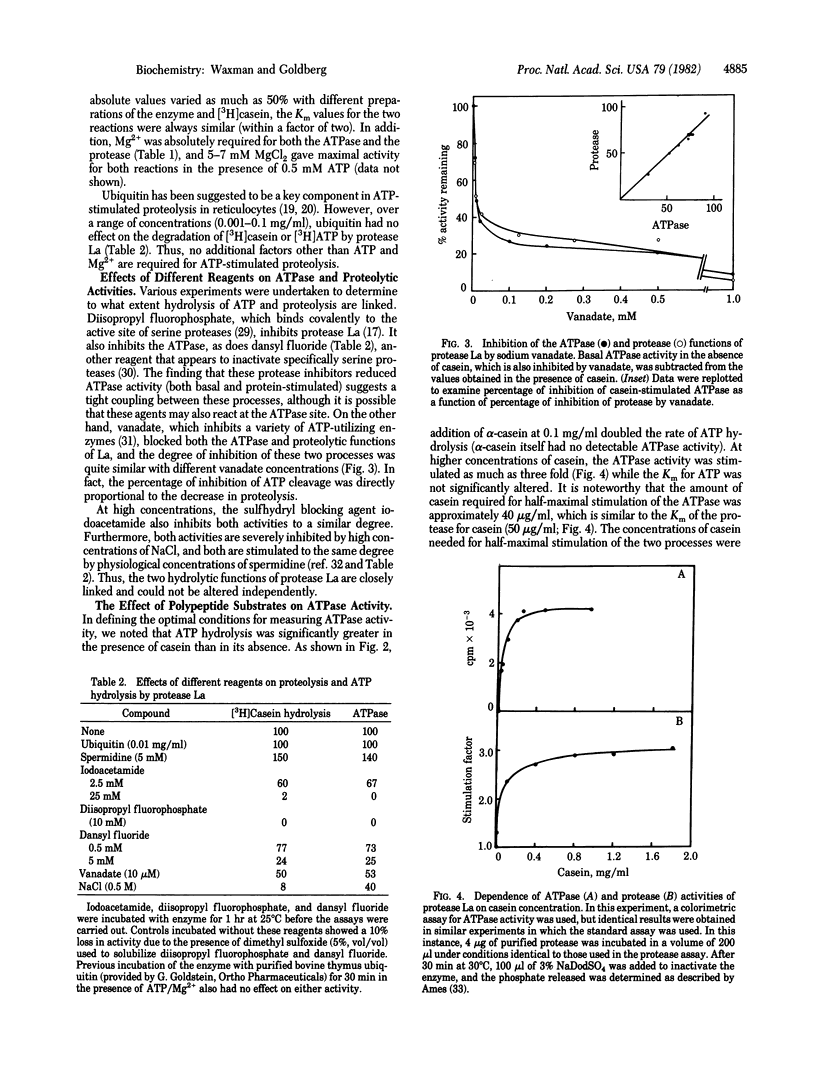
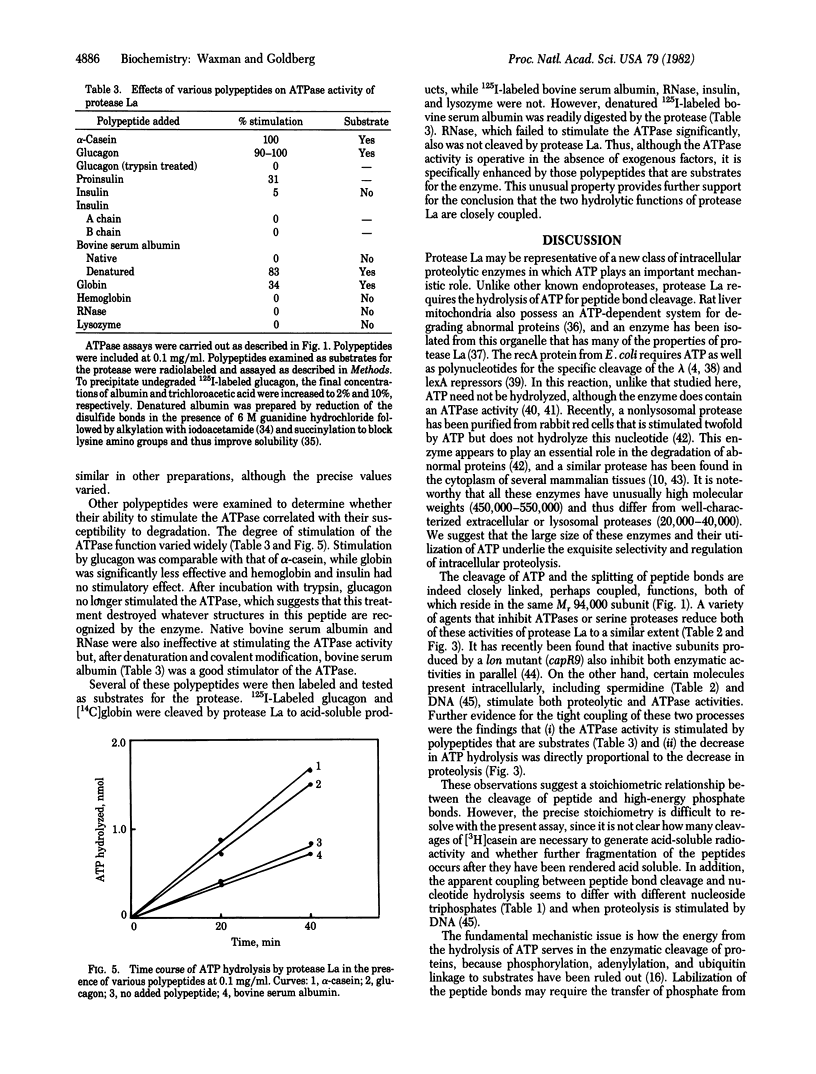
Abstract
The energy requirement for protein breakdown in Escherichia coli results from an ATP requirement for the function of protease La, the product of the lon gene. This novel serine protease contains an ATPase activity that is essential for proteolysis. ATP and protein hydrolysis show the same Km for ATP (30-40 muM) and are affected similarly by various inhibitors, activators, and ATP analogs. Vanadate inhibited ATP cleavage and caused a proportionate reduction in casein hydrolysis, and inhibitors of serine proteases reduced ATP cleavage. Thus, ATP and protein hydrolysis appear to be linked stoichiometrically. Furthermore, ATP hydrolysis is stimulated two- to threefold by polypeptides that are substrates for the protease (casein, glucagon) but not by nonhydrolyzed polypeptides (insulin, RNase). Unlike hemoglobin or native albumin, globin and denatured albumin stimulated ATP hydrolysis and were substrates for proteolysis. It is suggested that the stimulation of ATP hydrolysis by potential substrates triggers activation of the proteolytic function.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belhadj O., Sentenac A., Fromageot P. RNA-dependent ATPase from Saccharomyces cerevisiae. J Biol Chem. 1980 Dec 25;255(24):11704–11709. [PubMed] [Google Scholar]
- Bukhari A. I., Zipser D. Mutants of Escherichia coli with a defect in the degradation of nonsense fragments. Nat New Biol. 1973 Jun 20;243(129):238–241. doi: 10.1038/newbio243238a0. [DOI] [PubMed] [Google Scholar]
- Charette M. F., Henderson G. W., Markovitz A. ATP hydrolysis-dependent protease activity of the lon (capR) protein of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4728–4732. doi: 10.1073/pnas.78.8.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. H., Goldberg A. L. DNA stimulates ATP-dependent proteolysis and protein-dependent ATPase activity of protease La from Escherichia coli. Proc Natl Acad Sci U S A. 1982 Feb;79(3):795–799. doi: 10.1073/pnas.79.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. H., Goldberg A. L. The product of the lon (capR) gene in Escherichia coli is the ATP-dependent protease, protease La. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4931–4935. doi: 10.1073/pnas.78.8.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A., Heller H., Elias S., Haas A. L., Hershko A. ATP-dependent conjugation of reticulocyte proteins with the polypeptide required for protein degradation. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1365–1368. doi: 10.1073/pnas.77.3.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig N. L., Roberts J. W. E. coli recA protein-directed cleavage of phage lambda repressor requires polynucleotide. Nature. 1980 Jan 3;283(5742):26–30. doi: 10.1038/283026a0. [DOI] [PubMed] [Google Scholar]
- Desautels M., Goldberg A. L. Liver mitochondria contain an ATP-dependent, vanadate-sensitive pathway for the degradation of proteins. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1869–1873. doi: 10.1073/pnas.79.6.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterbrook-Smith S. B., Wallace J. C., Keech D. B. Pyruvate carboxylase: affinity labelling of the magnesium adenosine triphosphate binding site. Eur J Biochem. 1976 Feb 2;62(1):125–130. doi: 10.1111/j.1432-1033.1976.tb10105.x. [DOI] [PubMed] [Google Scholar]
- Etlinger J. D., Goldberg A. L. A soluble ATP-dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes. Proc Natl Acad Sci U S A. 1977 Jan;74(1):54–58. doi: 10.1073/pnas.74.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federation of American Societies for Experimental Biology. 66th Annual meeting. New Orleans, Louisiana, April 15-23, 1982. Abstracts of papers 3478-6993. Fed Proc. 1982 Mar 5;41(4):865–1466. [PubMed] [Google Scholar]
- Goldberg A. L. Correlation between rates of degradation of bacterial proteins in vivo and their sensitivity to proteases. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2640–2644. doi: 10.1073/pnas.69.9.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Dice J. F. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem. 1974;43(0):835–869. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Strnad N. P., Swamy K. H. Studies of the ATP dependence of protein degradation in cells and cell extracts. Ciba Found Symp. 1979;(75):227–251. doi: 10.1002/9780470720585.ch15. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Swamy K. H., Chung C. H., Larimore F. S. Proteases in Escherichia coli. Methods Enzymol. 1981;80(Pt 100):680–702. doi: 10.1016/s0076-6879(81)80052-3. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Zipser D. Deg phenotype of Escherichia coli lon mutants. J Bacteriol. 1978 Feb;133(2):844–851. doi: 10.1128/jb.133.2.844-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. 5-Oxo-L-prolinase (L-pyroglutamate hydrolase). Studies of the chemical mechanism. J Biol Chem. 1981 Oct 10;256(19):9981–9985. [PubMed] [Google Scholar]
- HARTLEY B. S. Proteolytic enzymes. Annu Rev Biochem. 1960;29:45–72. doi: 10.1146/annurev.bi.29.070160.000401. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A., Heller H., Haas A. L., Rose I. A. Proposed role of ATP in protein breakdown: conjugation of protein with multiple chains of the polypeptide of ATP-dependent proteolysis. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1783–1786. doi: 10.1073/pnas.77.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer H., Heinrich P. C. Control of proteolysis. Annu Rev Biochem. 1980;49:63–91. doi: 10.1146/annurev.bi.49.070180.000431. [DOI] [PubMed] [Google Scholar]
- Kowit J. D., Goldberg A. L. Intermediate steps in the degradation of a specific abnormal protein in Escherichia coli. J Biol Chem. 1977 Dec 10;252(23):8350–8357. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larimore F. S., Waxman L., Goldberg A. L. Studies of the ATP-dependent proteolytic enzyme, protease La, from Escherichia coli. J Biol Chem. 1982 Apr 25;257(8):4187–4195. [PubMed] [Google Scholar]
- Little J. W., Edmiston S. H., Pacelli L. Z., Mount D. W. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W. The genetics of protein degradation in bacteria. Annu Rev Genet. 1980;14:279–319. doi: 10.1146/annurev.ge.14.120180.001431. [DOI] [PubMed] [Google Scholar]
- Murakami K., Voellmy R., Goldberg A. L. Protein degradation is stimulated by ATP in extracts of Escherichia coli. J Biol Chem. 1979 Sep 10;254(17):8194–8200. [PubMed] [Google Scholar]
- Ogawa T., Wabiko H., Tsurimoto T., Horii T., Masukata H., Ogawa H. Characteristics of purified recA protein and the regulation of its synthesis in vivo. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):909–915. doi: 10.1101/sqb.1979.043.01.099. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Craig N. L., Phizicky E. M. Activity of the Escherichia coli recA-gene product. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):917–920. doi: 10.1101/sqb.1979.043.01.100. [DOI] [PubMed] [Google Scholar]
- Swamy K. H., Goldberg A. L. E. coli contains eight soluble proteolytic activities, one being ATP dependent. Nature. 1981 Aug 13;292(5824):652–654. doi: 10.1038/292652a0. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Vaz W. L., Schoellmann G. Specific fluorescent derivatives of macromolecules. Reaction of dansyl fluoride with serine proteinases. Biochim Biophys Acta. 1976 Jul 19;439(1):194–205. doi: 10.1016/0005-2795(76)90175-6. [DOI] [PubMed] [Google Scholar]
- Voellmy R. W., Goldberg A. L. ATP-stimulated endoprotease is associated with the cell membrane of E. coli. Nature. 1981 Apr 2;290(5805):419–421. doi: 10.1038/290419a0. [DOI] [PubMed] [Google Scholar]
- Yount R. G. ATP analogs. Adv Enzymol Relat Areas Mol Biol. 1975;43:1–56. doi: 10.1002/9780470122884.ch1. [DOI] [PubMed] [Google Scholar]
- Zehnbauer B. A., Foley E. C., Henderson G. W., Markovitz A. Identification and purification of the Lon+ (capR+) gene product, a DNA-binding protein. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2043–2047. doi: 10.1073/pnas.78.4.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werf P., Meister A. The metabolic formation and utilization of 5-oxo-L-proline (L-pyroglutamate, L-pyrrolidone carboxylate). Adv Enzymol Relat Areas Mol Biol. 1975;43:519–556. doi: 10.1002/9780470122884.ch7. [DOI] [PubMed] [Google Scholar]



