Abstract
Background:
Probably microbial plaque is the main etiology for periodontal tissue inflammation. Various chemical agents have been evaluated over the years with respect to their antimicrobial effects in the oral cavity. However, all are associated with side effects that prohibit regular long-term use. Therefore, the effectiveness of Azadirachta indica (neem) against plaque formation is considered to be vital, with lesser side effects. The aim of the present study is to evaluate and prove the antimicrobial activity of neem using plaque samples.
Materials and Methods:
Culture was prepared using brain heart infusion broth reagent. Dental plaque samples were used for that. Kirby–Bauer antimicrobial susceptibility test procedure was carried away with the sample. Neem oil was kept in the agar plate with culture and the diameter of inhibition zones was calculated.
Results:
Results showed inhibition zones on the agar plate around neem oil.
Conclusion:
Study shows definite antiplaque activity of neem oil.
KEY WORDS: Antimicrobial, anti-plaque agents, herbal, neem
Periodontal diseases are the most prevalent oral health problems caused by dental plaque. Dental plaque is a complex microbial community with greater than 108 bacteria per mg. It is estimated that as many as 400 distinct bacterial species may be found in plaque. Recently, specific microorganisms in plaque have been implicated in chronic periodontal diseases.[1]
A dynamic equilibrium between the periodontal microbiota and host generally results in a clinical state of periodontal health, characterized by minimal inflammatory changes in marginal gingival tissues. Maintenance of health is mostly achieved by controlling the residual mass of microorganisms.[1] The use of Azadirachta indica (Indian neem) is considered as the plants have a wide spectrum of bioactivity. They are used as antibacterial, antifungal, and anticancerous agents.[2] They had shown good broad range of antibacterial activity in vitro (Rae et al. 1986). The purpose of the present study is to utilize this Indian herb in the reduction of microorganisms in the dental plaque.
Aims and Objectives
To evaluate the effects of neem oil on plaque microorganisms
To correlate the natural product's significant effect in reduce microorganisms in plaque when treated with untreated plaque.
Materials and Methods
Preparation of culture
Culture was prepared using brain heart infusion broth reagent. Thirty-seven grams of brain heart infusion broth was weighed and suspended in 1000 ml of distilled water. Twenty milliliters of the suspension was dispersed in five bottles. Then, the bottles were autoclaved at 15 lbs pressure (121°C) for 15 min.
Sample collection
After obtaining IEC approval, a study population of five patients was randomly selected from the Department of Periodontics. Plaque removed from the patients was utilized as the sample and collected in culture bottles.
This culture was incubated at 37°C for 48 h. After 48 h, colonies of microbial growth were noted.
Kirby–Bauer antimicrobial susceptibility test procedure
The main purpose of performing Kirby–Bauer test[3] is for the evaluation of antimicrobial activity of chemotherapeutic agents. A standardized filter paper disk agar diffusion procedure, known as the Kirby–Bauer method, is frequently used to determine the drug susceptibility of microorganisms isolated from infectious processes. It allows for the rapid determination of the efficacy of a drug by measuring the diameter of the zone of inhibition that results from diffusion of the agent into the medium surrounding the disk. In this procedure, filter paper disks of uniform size are impregnated with specified concentrations of different antibiotics and then placed on the surface organism to be treated. Mueller-Hinton agar, the medium of choice with a pH range of 7.2–7.4, is poured into plates to a uniform depth of 5 mm and refrigerated on solidification. Prior to use, the plates are transferred to an incubator at 37°C for 10--20 min to dry the moisture that develops on the agar surface.
The plates are then heavily inoculated with standardized inoculums by means of a cotton swab to ensure the confluent growth of the organism. The disks are aseptically applied on the surface of the agar plate at well-spaced intervals. Once applied, each disk is gently touched with a sterile applicant stick to ensure its firm contact with agar surface.
Following incubation, the plates are examined for the presence of growth inhibition, which is indicated by a clear zone surrounding each disk. The susceptibility of an organism to a drug is determined by the size of the zone, which itself is dependent on variables such as:
Ability and rate of diffusion of the antibiotic into the medium and its interaction with the test organism,
The number of organisms inoculated,
The growth rate of the organism, and
The degree of susceptibility of the organism to the antibiotic.
A measurement of diameter of the zone of inhibition in millimeters is made and its size is compared. Based on this comparison, the test organism is determined to be resistant, intermediate, or susceptible to the antibiotic. More than the range of 17–20 mm inhibition zone is considered as susceptible to antibiotics.
5% concentration of neem oil was tried in this study. Agar plates were placed right side up in an incubator heated to 37°C for 10–20 min. A sterile cotton swab was dipped into a well-mixed test culture of one patient and excess inoculums were removed by pressing the saturated swab against the inner wall of culture tube. Onto the agar surface, the swab was streaked horizontally, vertically, and around the outer edge of the plate to ensure a heavy growth over the entire surface. All the agar plates were allowed to dry for about 5 min. Using a dispenser, neem oil was applied on the agar surface. All five plates were incubated in an inverted position for 24–48 h at 37°C. Results were evaluated and compared. All five samples had clear inhibition zone with diameter range of 20–30 mm for 5% neem oil medium.
Results
After 48 h, inhibition zones were formed on the agar plates impregnated with neem oil. The inhibition zone signifies the reduction of microorganisms on the agar plate [Figures 1 and 2]. These inhibition zones on the agar plates looked circular in shape and the zones were measured with the help of a millimeter scale [Table 1].
Figure 1.
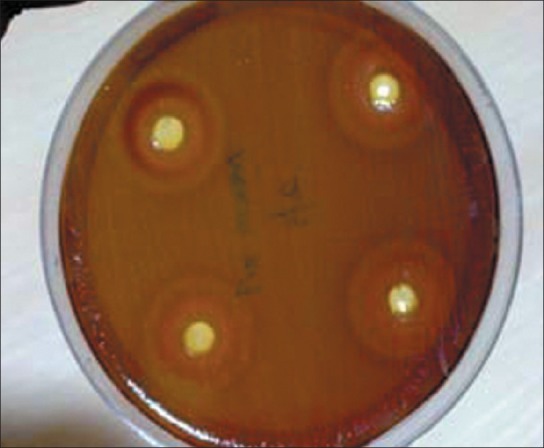
Inhibition zones formed on agar when neem oil was diffused
Figure 2.
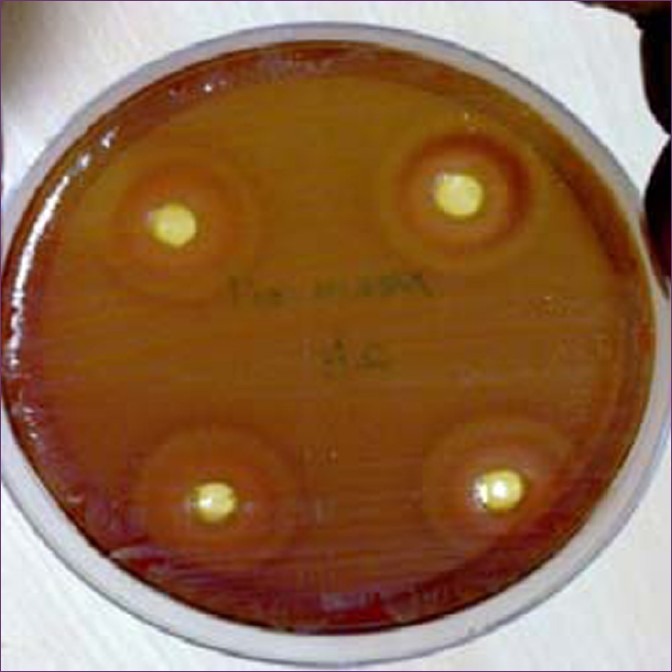
Inhibition zones formed on agar when neem oil was diffused
Table 1.
Size of the inhibition zones

Discussion
Periodontal disease is a general term which encompasses several pathological conditions affecting the tooth supporting structure The clinical signs of periodontitis are changes in the morphology of gingival tissues, bleeding upon probing, as well as periodontal pocket formation. This periodontal pocket provides an ideal environment for the growth and proliferation of anaerobic pathogenic bacteria.[1]
Dental plaque is a highly specific variable structural entity formed by sequential colonization of microorganisms on the tooth surface. It is a structured, resilient, yellow-grayish substance that adheres tenaciously to intraoral hard substance. One gram of plaque contains nearly 100,000,000,000 bacteria.
Anti-plaque agents prevent the accumulation of plaque on the tooth surface. The efforts of dental researchers have resulted in the availability of wide range of anti-plaque agents, thus preventing the accumulation of plaque. The available antimicrobial agents produce resistant strains. Chlorhexidine mouthwash may stain the teeth. The use of herbs as antimicrobial produces no side effects, and they are easily available to the rural population at a low cost.[4]
Neem tree (A. indica) was described as early as in 1830 by De Jussieu.[5] It belongs to Meliaceae family. Neem is an evergreen tree cultivated in Indian subcontinent and is popularly known as “Indian neem” or “Indian lilac.” Every part of the tree has been used in traditional medicine for household remedy.[6,7] Bioactive compounds in neem include: nimbidin, nimbolide, gedunin, and mahmoodin. Nimbidin has anti-inflammatory, anti-arthritic, antipyretic, hypoglycemic, and anti-bacterial property.[8,9] It is active against Klebsiella, Staphylococcus, and Serratia species. It is also active against Streptococcus mutans and Streptococcus faecalis.[10]
Reduction of microorganisms is confirmed by the formation of inhibition zones on Mueller-Hinton agar plates. The microorganisms to be tested are obtained from the plaque samples cultured in the brain heart infusion broth. Colonies of microorganisms are formed in the media when incubated for 48 h. When the culture is inoculated onto the Mueller-Hinton agar and neem oil is diffused, it shows formation of inhibition zones. The formation of inhibition zones takes place after 48 h. Henceforth, neem oil can be used to control periodontal diseases and limit the progression of periodontitis.
Conclusion
This in vitro study concludes that the commonly used natural herbs among the rural people, such as neem, have beneficial antimicrobial activity. Hence, further studies are required in formulating the anti-plaque agents based upon herbs like A. indica.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Vaidya ADB, Devasagayam TP. Current Status of Herbal Drugs in India: An Overview. J Clin Biochem Nutr. 2007;41:1–11. doi: 10.3164/jcbn.2007001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhowmik D, Chiranjib, Yadav J, Tripathi KK, SampathKumar KP. Herbal remedies of Azadirachta indica and its medicinal application. J Chem Pharm Res. 2010;2:62–72. [Google Scholar]
- 3.Biswas K, Chattopadhyay I, Banerjee RK, Bandyopadhyay U. Biological activities and medicinal properties of neem (Azadirachta indica) Curr Sci. 2002;8:1336–45. [Google Scholar]
- 4.Boyle J, Fancher ME, Ross RW., Jr Rapid Modified Kirby-Bauer Susceptibility Test with single, high-concentration antimicrobial disks. Antimicrob Agents Chemother. 1973;3:418–24. doi: 10.1128/aac.3.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee A, Pakrashi SC. The Treatise on Indian Medicinal Plants, Volume 2. Publications & Information Directorate, 1992. 1994;3:76. [Google Scholar]
- 6.Chopra RN, Nayer SL, Chopra IC. Glossary of Indian Medicinal plants. New Delhi: CSIR; 1956. [Google Scholar]
- 7.Drabu S, Khatri S, Babu S. Neem: Healer of All Ailments. Res J Pharm Biol Che Sci. 2012;3:120–6. [Google Scholar]
- 8.Biswas K, Chattopadhyay I, Banerjee RK, Bandyopadhyay U. Biological activities and medicinal properties of neem (Azadirachta indica) Cur Scien. 2002;8:1336–45. [Google Scholar]
- 9.Marsh PD. Host defences and microbial homeostasis: role of microbial interactions. J Dent Res. 1989;68:1567–75. [Google Scholar]
- 10.Subapriya R, Nagini S. Medicinal properties of neem leaves: A review. J Ethnopharmacol. 2003;88:107–11. doi: 10.2174/1568011053174828. [DOI] [PubMed] [Google Scholar]


