Abstract
Objective:
To quantify the adherence of Streptococcus mutans and Candida albicans on brackets made of stainless steel, plastic, ceramic, titanium, and gold, and to evaluate the various sites of adherence of these microorganisms with scanning electron microscopy (SEM).
Materials and Methods:
Brackets made of stainless steel, plastic, ceramic, titanium, and gold were used. The adherence of S. mutans and C. albicans were studied. The brackets were placed in flat-bottomed vials containing basal medium with 20% sucrose added; the flasks were inoculated with each of the microbial suspensions. The samples were incubated at 37°C for 48 h, after which the brackets were removed. The cells adhering to the glass were counted and the brackets were studied with SEM.
Results:
When evaluated together, the adherence of S. mutans and C. albicans was increased in the ceramic bracket group. When evaluated separately, metallic brackets had increased number of colony-forming units (CFUs) of S. mutans and the use of titanium brackets increased the CFUs of C. albicans. SEM demonstrated that the adherence of S. mutans and C. albicans together varied according to the bracket materials, with ceramic having the greatest and stainless steel having the least adherence.
Conclusions:
Oral hygiene may be of greater concern with esthetic brackets since this study shows that microbial adhesion is greater with these brackets.
KEY WORDS: Adherence, brackets candida, streptococcus
For a long time, the traditional orthodontic patient was considered as a low-risk patient and orthodontic procedures were considered as noninvasive. However, the placement of these fixed orthodontic appliances (FOAs) in the oral cavity results in iatrogenic side effects such as bacteraemia.[1–3] Orthodontic treatment with fixed appliances leads to an increased amount of plaque accumulation and elevated levels of Streptococcus mutans and lactobacilli which are considered to be the main pathogens in the development of dental caries.[4]
Yeasts of the Candida species were analyzed because they are the frequently found microorganisms in the oral cavity. This yeast has been proven to colonize on cement, enamel, and dentin, which serve as a reservoir for the spread of the microorganism.[5] Nevertheless, the yeast's ability to survive on inert surfaces needs to be further studied in order to understand its virulence and dissemination routes.[6] S. mutans was studied because of its well-documented role in the pathogenesis of caries.[7] Although a number of studies have demonstrated the viability of Candida albicans and S. mutans on removable orthopedic appliances, little is known about their survival on FOAs.[8]
The aim of this study was to quantify the adherence of S. mutans and C. albicans on stainless steel, plastic, ceramic, titanium, and gold brackets, and to evaluate various sites of adherence of these microorganisms on these bracket materials with scanning electron microscopy (SEM).
Materials and Methods
Brackets
Brackets used were first premolar brackets of the 022 × 028 Roth prescription, made of five different materials. They were divided into the following six groups which include a control group which did not have any brackets:
Group I: Stainless steel brackets (Victory 3M, Unitek, Monrovia, CA, USA)
Group II: Composite brackets (Brilliant, Forestadent, Pforzehim, Germany)
Group III: Ceramic brackets (Clarity, 3M, Monrovia, CA, USA).
Group IV: Titanium brackets (Orthos, Ormco, Glendora, CA, USA)
Group V: Gold brackets (Magnum, Orthodontic Design and Production, Inc., Vista, CA, USA)
Group VI: Control, without brackets.
In each group, 24 samples for culture were taken as follows:
8 containing S. mutans,
8 containing C. albicans, and
8 containing a combination of S. mutans and C. albicans.
Microorganisms
The microorganisms used were a wild strain of S. mutans provided by MicroBioLogics, Inc, Minnesota, USA, an ATCC 35668 strain of S. mutans, an oral stain of C. albicans isolated from the Sri Ramachandra University Microbiology Laboratory, and an ATCC 10231 strain. S. mutans was seeded in brain heart infusion agar under microanaerobiosis at 37°C for 24 h. C. albicans was seeded in Sabouraud Dextrose Agar and incubated under aerobic condition at 37°C for 18 h. Microbial suspensions were prepared with each species on reaching the exponential growth.
Experimental test
Eight samples from each of the five experimental groups were cultured with one of the microorganism groups. The procedure was performed as suggested by Brusca et al.[9] with flat-bottomed vials.
The brackets were then transferred to the respective medium with 20% of sucrose added to it. Sucrose was used because S. mutans produces glucans from sucrose, which allows the adherence to the glass surface of the vials. Eight vials of each group were analyzed. These eight vials of each group were inoculated with 1 ml of microbial solution in a concentration of 1 × 105 and 1 × 107 colony-forming units (CFUs) of mutans streptococci and C. albicans, respectively. This was achieved by matching the culture media with the organism to the control with the help of turbidity meter, Dade Behering, MicroScan, Dade Behring Microscan Inc West Sacramento, CA according to MacFarlane standard. Then, the brackets were placed in the glass vials with the help of straight point tweezers. The control group consisted of three vials, one with S. mutans, one with C. albicans, and the third tube with both S. mutans and C. albicans. These vials did not have any brackets in them. This represented the control group.
The procedure was performed close to a culture oven and the vials were placed in a crate made ad hoc to secure them in a fixed position. The samples were incubated at 37°C for 48 h. The brackets were removed using straight point tweezers. The supernatant in the vials was removed, the vials were turned upside down to perform the macroscopic examination of the cells adhered to the glass, and specially designed score grid was placed on the bottom of the vials. After the bracket removal, each of the experimental vials was compared with the control vials to evaluate the adherence to the brackets.
The brackets were then processed for electron microscopy, during which they were dehydrated in alcohol, dried, and fixed in 10% formaldehyde and treated with gold palladium using HITACHI E-1010 ion sputter. The brackets were then observed using HITACHI S - 3400 scanning microscope with ×50 to ×2500 magnifications.
The biological controls included commercial strip with bacterial spores containing 1.6 × 104 Bacillus stearothermophilus and 2.3 × 104 Bacillus subtilisvarniger. The viability of the spore in the strips was tested prior to the study using staining techniques that allow for morphological evaluation.
Statistical analysis
The results were expressed as the number of CFUs per milliliter (CFU/ml). Statistical analysis was done with SPSS software version 15 and the results were expressed as mean values. One-way analysis of variance (ANOVA) and Scheffé post hoc test were used for multiple comparisons. A value of P<0.05 was considered significant.
Results
The results obtained under these experimental conditions showed that the capacity of each of the studied microorganisms to adhere to glass and brackets was determined.
Table 1 shows the number of CFUs adhering to the glass surface of the flat-bottomed vials without brackets. S. mutans exhibited the highest CFU number, whereas C. albicans exhibited the lowest. Both species added together resulted in a significant decrease in CFU/ml, compared with S. mutans alone (P<0.001) [Table 1].
Table 1.
CFUs adhering to the glass surface of the flat-bottomed vials without brackets

Discussion
One of the common problems that should be avoided in orthodontic treatment is the development of dental plaque which is initiated by the adhesion of S. mutans to the tooth surface or orthodontic devices. Ervedi[2] stated that most orthodontic patients are not able to perform effective plaque control, and therefore develop mild to moderate gingivitis during treatment with fixed appliances. It is accepted that the presence of FOAs greatly inhibits oral hygiene and creates new retentive areas for plaque and debris, which in turn predisposes to increased carriage of microbes and subsequent infection.[10] Hagg et al.[10] discussed a link between an increased level of dental plaque in individuals treated with FOAs and the subsequent development of gingivitis. Hamp et al.[11] have shown that subjects with FOA have a loss of periodontal support. Ogaard[12] has indicated an increasing incidence of incipient carious lesion on the facial and lingual aspects of the teeth, while Rosenbloom and Tinanoff[13] reported increased Gram-positive bacterial counts in saliva during treatment with FOAs.
Plaque accumulating around orthodontic brackets often results in enamel white spot formation adjacent to brackets. This plaque is composed of various microorganisms of which S. mutans is the most cariogenic. Its adherence to the fixed appliance is largely contributed by the bracket material.
S. mutans along with glycosyltransferase degrades sucrose to make insoluble glucans. These insoluble glucans also attach to the tooth surface, providing ideal sites for oral bacteria to inhabit. The resulting complex of glucan and various bacteria then creates an oral biofilm which is the mature stage of dental plaque. As plaque accumulates, acidic compounds such as fructose and other fatty acids degrade the enamel surface of the teeth through a process known as dental caries.
Hagg et al.[10] discussed the high oral colonization by the fungal pathogen C. albicans in individuals wearing either full or partial removable denture. Candida species have also been isolated from dental plaque and caries and the subgingival flora.[10] Sen et al.[14] isolated Candida species from human dental hard tissue. Nevertheless, as yet, there have been limited studies examining the colonization trends of Candida species in well-defined populations using FOAs.[10]
Evaluating S. mutans alone revealed that there was a greater number of CFUs on stainless steel brackets than on plastic and ceramic brackets. This finding is in concurrence with that of Ahn et al.,[15] but not in agreement with the reports of Papaioannou et al.,[16] Fournier et al.,[17] and Brusca et al.,[9] who found no obvious difference in the adhesion of S. mutans to stainless steel, plastic, and ceramic brackets. Titanium and gold brackets also showed lesser CFUs than stainless steel brackets.
When C. albicans was evaluated alone, the results revealed a greater adherence to plastic and ceramic brackets than to stainless steel brackets, and this finding is in accordance with the report of Brusca et al.[9] Gold brackets had the least number of CFUs and were similar to the control group [Figure 1]. This could be because of the inert properties of gold. Titanium brackets had the greatest number of CFUs and this could be because of the rough surface characteristics of this bracket.[18]
Figure 1.
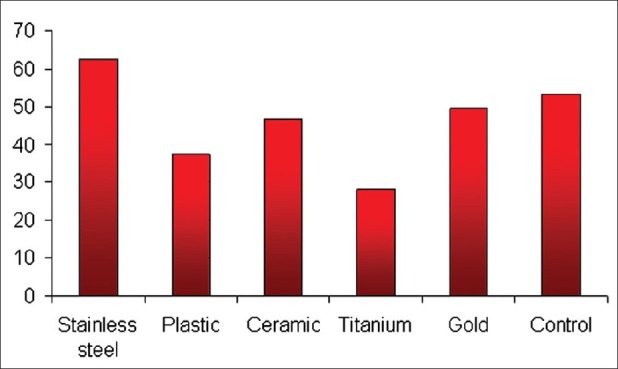
shows quantitatively that the number of CFUs of S. mutans was modified in the presence of different bracket types. The mean CFUs/ml of S. mutans for stainless steel brackets was found to be the highest, followed by gold, ceramic, plastic, and titanium in descending order
When S. mutans and C. albicans were evaluated in combination, which is more reflective of the clinical situation, rather than individual examination of the microorganisms, there was a decrease in CFUs of both S. mutans and C. albicans, revealing an antagonistic relationship at least in the initial growth. This finding is in accordance with the Brusca et al.[9] who also reported that once the microorganisms are established in the plaque, they do not inhibit each other, but rather seem to exert a synergistic effect. There was a greater number of CFUs for plastic and ceramic brackets when compared to the metal brackets, with stainless steel having the least number of CFUs.[17]
Examining the brackets showed that titanium had the least number of CFUs with S. mutans alone, while it had the greatest number of CFUs with C. albicans alone but was similar to stainless steel brackets when the organisms were evaluated together [Figure 2]. This could suggest some antibacterial property of titanium to S. mutans, which was not effective against the fungi, thus mirroring Bundy's[19] observation of titanium being an inhibitor, but this property not being consistent.
Figure 2.
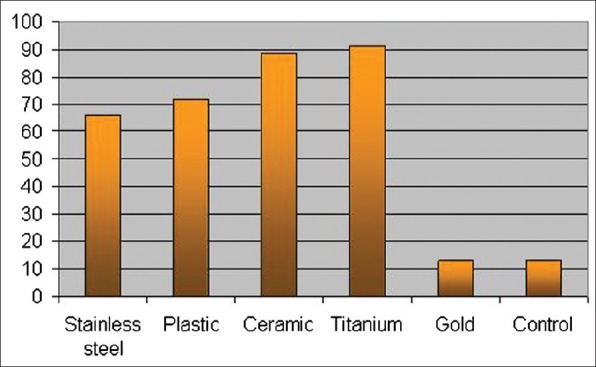
shows comparatively the adherence of C. albicans among the bracket types. It can be seen that the number of CFUs differs according to the bracket composition. The adherence of C. albicans was similar in the case of titanium and plastic brackets, with titanium having the highest count. Following plastic were stainless steel, gold, and composite brackets in descending order. The gold brackets were similar to the controls in case of S. mutans and C. albicans
Since the fungi grow by hyphae formation, the rough surface characteristics of titanium brackets could have facilitated the increased levels of C. albicans when it was studied alone.[18] Gold brackets showed a decreased number of CFUs when S. mutans and C. albicans were analyzed alone or in combination. This could be because of the inert properties of gold. Plastic and ceramic brackets showed a decreased number of CFUs when S. mutans was evaluated alone and the levels were increased when C. albicans was studied alone and when the organisms were evaluated together. Stainless steel brackets showed increased levels when S. mutans was evaluated separately, but decreased levels with C. albicans and when the organisms were evaluated in combination [Figure 3].
Figure 3.
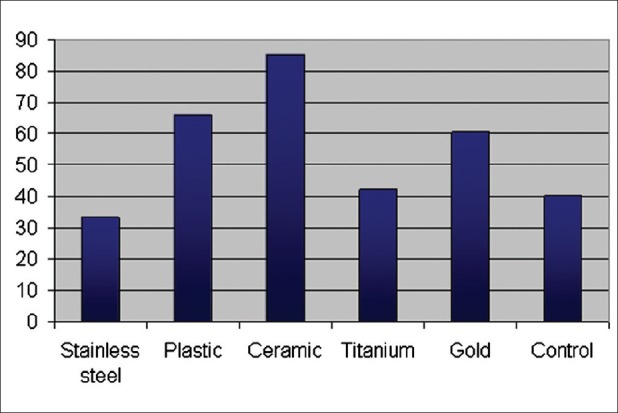
shows the CFUs/ml when S. mutans and C. albicans were added together. A decrease in CFUs/ml was observed in the presence of metallic brackets. And to the contrary, the ceramic brackets triggered an increase in CFU/ml. The titanium brackets were similar to the control in the number of CFU/ml
The variation between brackets when S. mutans was analyzed alone could be explained on the basis of surface free energy and tension. Van Dijk[20] has shown that more microorganisms adhered to substrata with an initially high surface free energy than to substrata with an initially low surface free energy. Eliades[21] has shown that stainless steel had the highest critical surface tension, indicating an increased potential for microorganism attachment on metallic brackets. Accordingly, a material with high surface free energy will attract more bacteria to its surface than a material with low surface free energy [Figures 4–13].[20] Eliades et al.[21] have suggested that metal brackets increase the level of bacterial adhesion because of the greater surface energy compared with ceramic brackets.
Figures 4–13.
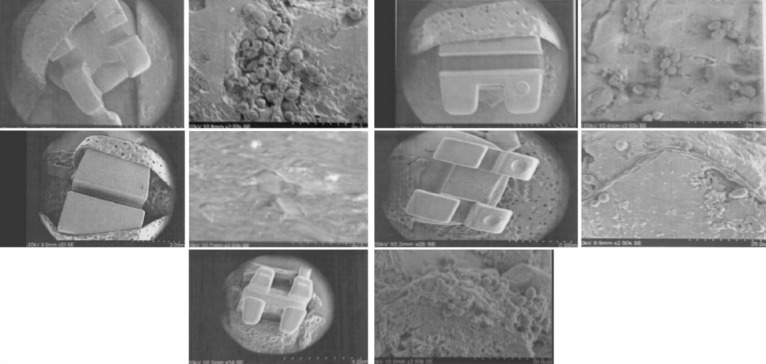
show the electron microscopy images of the different brackets (stainless steel, composite, ceramic, titanium, and gold) removed from the vials containing S. mutans and C. albicans together. It can be seen that the adherence of microorganisms to the brackets was great on the slot zone and varied according to bracket composition. It was higher on the ceramic and lower on the metallic brackets
Evaluation under SEM showed that the yeasts exhibited numerous cell elongations in the presence of composite, which could be interpreted as potential intercellular bridges involved in the adhesion mechanism that allows the formation of pseudohyphae. The formation of pseudohyphae is noteworthy although the culture medium does not induce filamentation of C. albicans. Hyphae formation has been considered a virulence factor associated with greater invasive capacity, tissue invasion, and greater resistance to phagocytes.[9]
The present study is to evaluate in vitro the affinity of two important oral microorganisms, S. mutans and C. albicans, to various bracket materials which are used for a significant period of time during orthodontic treatment. However, various factors which could affect the microorganism colonization, like the presence of saliva, elastomers, metal ligatures, nickel, titanium, or steel arches, adhesives, etc., which are present in the oral cavity, warrant further in vivo studies to conclude the effects of microorganisms on appliance components.
Conclusions
When S. mutans and C. albicans were evaluated together, plastic and ceramic brackets showed greater number of CFUs than stainless steel, titanium, and gold.
When S. mutans was evaluated alone, stainless steel showed the greatest number of CFUs than any of the other bracket materials studied.
When C. albicans was evaluated alone, stainless steel showed the greatest number of CFUs than any of the other bracket materials studied.
When S. mutans and C. albicans were evaluated together, there was a decrease in CFUs, thereby indicating an antagonistic relation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Dajani AS, Taubert KA, Wilson W, Bolger AF, Bayer A, Ferrieri P, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA. 1997;277:1794–801. [PubMed] [Google Scholar]
- 2.Eliades T, Eliades G, Brantley WA. Microbial attachment on orthodontic appliances: I.Wettability and early pellicle formation on bracket materials. Am J Orthod Dentofacial Orthop. 1995;108:351–60. doi: 10.1016/s0889-5406(95)70032-3. [DOI] [PubMed] [Google Scholar]
- 3.Lee SJ, Kho HS, Lee SW, Yang WS. Experimental salivary pellicles on the surface of orthodontic materials. Am J Orthod Dentofacial Orthop. 2001;119:59–66. doi: 10.1067/mod.2001.110583. [DOI] [PubMed] [Google Scholar]
- 4.O’Reilly MM, Featherstone JDB. Demineralization and remineralization around orthodontic appliance: An in vivo study. Am J Orthod Dentofacial Orthop. 1987;92:33–40. doi: 10.1016/0889-5406(87)90293-9. [DOI] [PubMed] [Google Scholar]
- 5.Sen BH, Safavi KE, Spångberg LSW. Colonization of Candida albicans on cleaned human dental hard tissues. Arch Oral Bio. 1997;42:513–20. doi: 10.1016/s0003-9969(97)00026-5. [DOI] [PubMed] [Google Scholar]
- 6.Traoré O, Springthrope VS, Sattar S. A quantitative study of the survival of two species of Candida on porous and non-porous environmental surfaces and hands. J Appl Microbiol. 2002;92:549–55. doi: 10.1046/j.1365-2672.2002.01560.x. [DOI] [PubMed] [Google Scholar]
- 7.Gaidry D, Kudlick EM, Hutton JG, Russell DM. A survey to evaluate the management of orthodontic patients with a history of rheumatic fever or congenital heart disease. Am J Orthod. 1985;87:338–44. doi: 10.1016/0002-9416(85)90008-9. [DOI] [PubMed] [Google Scholar]
- 8.Arendroff T, Addy M. Candidal carriage and plaque distribution before, during and after removable orthodontic appliance therapy. J Clin Periodontol. 1985;12:360–8. doi: 10.1111/j.1600-051x.1985.tb00926.x. [DOI] [PubMed] [Google Scholar]
- 9.Brusca MI, Chara O, Sterin-Borda L, Rosa AC. Influence of different orthodontic brackets on the adherence of microorganisms in vitro. Angle Orthod. 2007;77:331–6. doi: 10.2319/0003-3219(2007)077[0331:IODOBO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Gwinnett AJ, Ceen RF. Plaque distribution on bonded brackets: A scanning microscope study. Am J Orthod. 1979;75:667–77. doi: 10.1016/0002-9416(79)90098-8. [DOI] [PubMed] [Google Scholar]
- 11.Hägg U, Kaveewatcharan ont P, Samaranayake YH, Samaranayake LP. The effect of fixed orthodontic appliances on the oral carriage of Candida species and enterobacteriacaece. Eur J Orthod. 2006;26:623–9. doi: 10.1093/ejo/26.6.623. [DOI] [PubMed] [Google Scholar]
- 12.Naranjo AA, Triviño ML, Jaramillo A, Betancourth M, Botero JE. Changes in the subgingivalmicrobiota and periodontal parameters before and 3 months after bracket placement. Am J Orthod Dentofacial Orthop. 2006;130:275, e17–22. doi: 10.1016/j.ajodo.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 13.Quiryen M, Bollen CML. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man A review of the literature. J Clin Periodontol. 1995;22:1–14. doi: 10.1111/j.1600-051x.1995.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 14.Scheie AA, Arneberg P, Krogstad O. Effect of orthodontic treatment on prevalence of Streptococcus mutans in plaque and saliva. Scand J Dent Res. 1984;92:211–7. doi: 10.1111/j.1600-0722.1984.tb00881.x. [DOI] [PubMed] [Google Scholar]
- 15.Ahn SJ, Lim BS, Yang CH, Chang YD. Quantitative analysis of the adhesion of cariogenic streptococci to orthodontic metal brackets. Angle Orthod. 2005;75:666–71. doi: 10.1043/0003-3219(2005)75[666:QAOTAO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.O’Sullivan JM, Howard F, Jenkinson HF, Cannon RD. Adhesion of Candida albicansto oral streptococci is promoted by selective adsorption of salivary proteins to the streptococcal cell surface. Microbiology. 2000;146:41–8. doi: 10.1099/00221287-146-1-41. [DOI] [PubMed] [Google Scholar]
- 17.Forsber GCM, Brattström V, Malmber GE, Nord CE. Ligature wires and elastomeric rings: Two methods of ligation, and their association with microbial colonization of Streptococcus mutans and lactobacilli. Eur J Orthod. 1991;13:416–20. doi: 10.1093/ejo/13.5.416. [DOI] [PubMed] [Google Scholar]
- 18.Kupietzky A, Majumdar AK, Shey Z, Binder R, Matheson PB. Colony forming unit levels of salivary Lactobacilli and Streptococcus mutans in orthodontic patients. J Clin Pediatr Dent. 2005;30:51–3. [PubMed] [Google Scholar]
- 19.Bundy KJ, Butler MF, Hochman RF. An investigation of the bacteriostatic properties of pure metals. J Biomed Mater Res. 1980;14:653–63. doi: 10.1002/jbm.820140511. [DOI] [PubMed] [Google Scholar]
- 20.Türkkahraman H, Sayın MÖ, Bozkurt FY, Yetkin Z, Kaya S, Önal S. Archwire ligation techniques, microbial colonization, and periodontal status in orthodontically treated patients. Angle Orthod. 2005;75:231–6. doi: 10.1043/0003-3219(2005)075<0227:ALTMCA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 21.de Soet JJ, van Loveren C, Lammens AJ, Pavicić MJ, Homburg CH, ten Cate JM, et al. Differences in cariogenicity between fresh isolates of Streptococcus sobrinus and Streptococcus mutans. Caries Res. 1991;25:116–22. doi: 10.1159/000261353. [DOI] [PubMed] [Google Scholar]


