Abstract
We have isolated lambda transducing phages carrying the Escherichia coli primase gene (dnaG) and mapped restriction sites in the cloned bacterial DNA segments. Several different DNA fragments containing the dnaG gene were inserted into multicopy plasmids. An analysis of the primase levels in cells harboring such plasmids indicates that sequences far upstream from the dnaG gene are required for optimal primase expression. Using this knowledge, we constructed a plasmid with a thermoinducible copy-number, pRLM61, which was employed to amplify intracellular primase levels approximately 100-fold. The dnaG gene is transcribed clockwise with respect to the E. coli genetic map, and a HindIII site located 180 base pairs upstream from the dnaG gene separates the gene from its primary promoter. An apparent transcription termination signal is positioned 30-70 base pairs in front of the primase gene. Transcription proceeds past this strong terminator only when RNA polymerase has first transcribed the bacterial DNA segment proximal to the HindIII site. We suggest that primase expression in E. coli is positively regulated by a mechanism of transcription antitermination mediated by a bacterial factor. We propose, furthermore, that the neighboring structural genes for primase and for the sigma subunit of RNA polymerase are coordinately regulated as part of an operon. This arrangement may enable the bacterial cell to readily control the level of initiation of DNA and RNA synthesis and thus to respond quickly and efficiently to changing conditions.
Full text
PDF
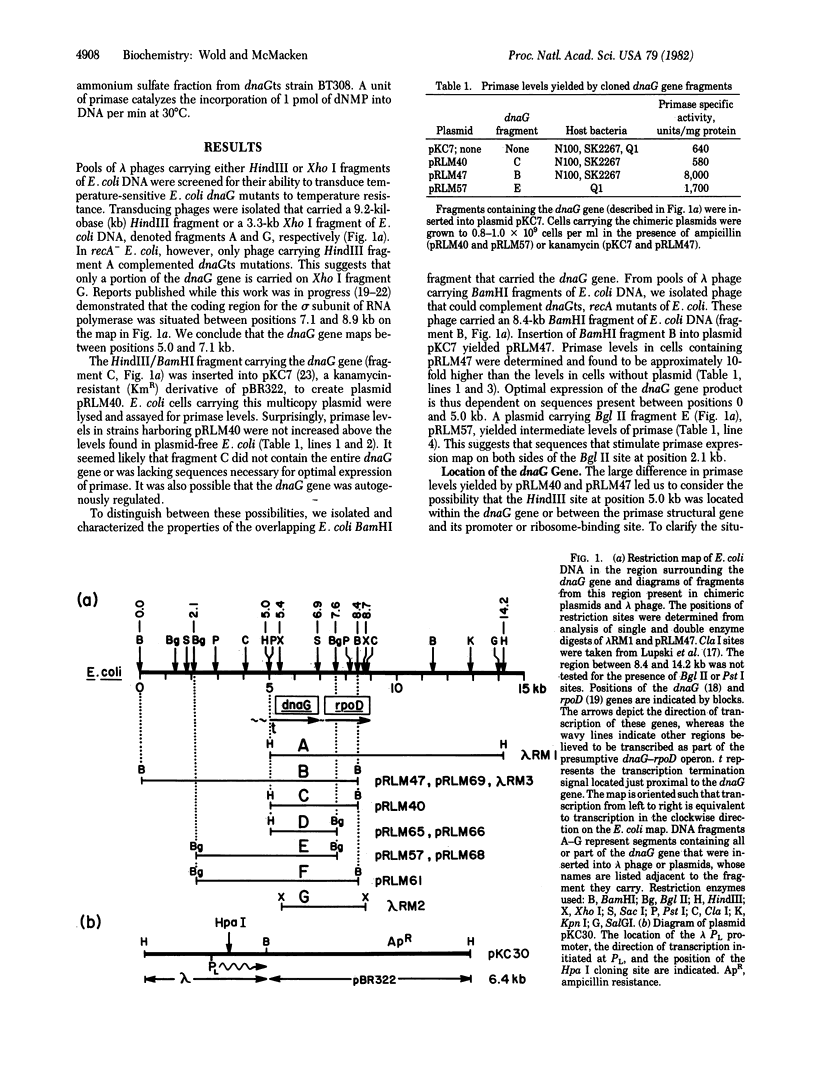

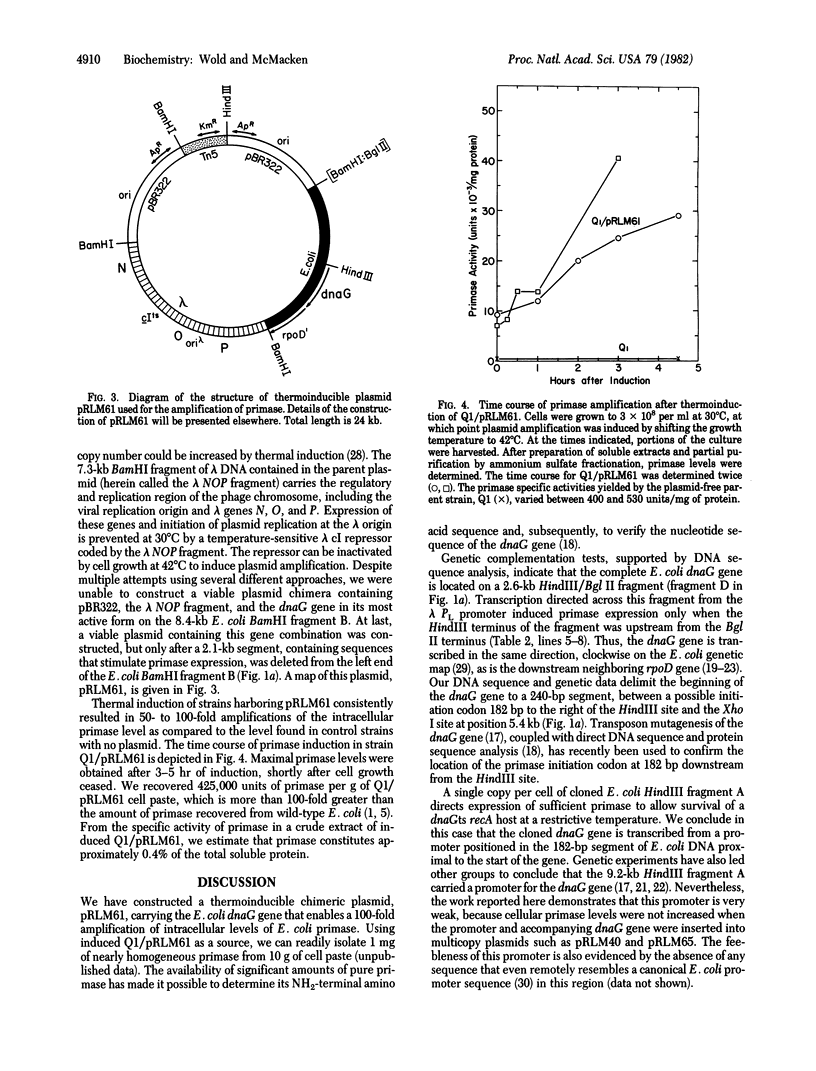

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Arai K., Kornberg A. Unique primed start of phage phi X174 DNA replication and mobility of the primosome in a direction opposite chain synthesis. Proc Natl Acad Sci U S A. 1981 Jan;78(1):69–73. doi: 10.1073/pnas.78.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman K., Ptashne M., Gilbert W. Construction of plasmids carrying the cI gene of bacteriophage lambda. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4174–4178. doi: 10.1073/pnas.73.11.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz E. W., Jr, Reinberg D., Vicuna R., Hurwitz J. Initiation of DNA replication by the dnaG protein. J Biol Chem. 1980 Feb 10;255(3):1096–1106. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton Z., Burgess R. R., Lin J., Moore D., Holder S., Gross C. A. The nucleotide sequence of the cloned rpoD gene for the RNA polymerase sigma subunit from E coli K12. Nucleic Acids Res. 1981 Jun 25;9(12):2889–2903. doi: 10.1093/nar/9.12.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralla J., Crothers D. M. Free energy of imperfect nucleic acid helices. II. Small hairpin loops. J Mol Biol. 1973 Feb 5;73(4):497–511. doi: 10.1016/0022-2836(73)90096-x. [DOI] [PubMed] [Google Scholar]
- Gross C. A., Blattner F. R., Taylor W. E., Lowe P. A., Burgess R. R. Isolation and characterization of transducing phage coding for sigma subunit of Escherichia coli RNA polymerase. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5789–5793. doi: 10.1073/pnas.76.11.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krell K., Gottesman M. E., Parks J. S., Eisenberg M. A. Escape synthesis of the biotin operon in induced lambda b-2 lysogens. J Mol Biol. 1972 Jul 14;68(1):69–82. doi: 10.1016/0022-2836(72)90263-x. [DOI] [PubMed] [Google Scholar]
- Low R. L., Arai K., Kornberg A. Conservation of the primosome in successive stages of phi X174 DNA replication. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1436–1440. doi: 10.1073/pnas.78.3.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupski J. R., Smiley B. L., Blattner F. R., Godson G. N. Cloning and characterization of the Escherichia coli chromosomal region surrounding the dnaG Gene, with a correlated physical and genetic map of dnaG generated via transposon Tn5 mutagenesis. Mol Gen Genet. 1982;185(1):120–128. doi: 10.1007/BF00333800. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McMacken R., Kornberg A. A multienzyme system for priming the replication of phiX174 viral DNA. J Biol Chem. 1978 May 10;253(9):3313–3319. [PubMed] [Google Scholar]
- Nakamura Y. Hybrid plasmid carrying Escherichia coli genes for the primase (dnaG) and RNA polymerase sigma factor (rpoD); gene organization and control of their expression. Mol Gen Genet. 1980;178(3):487–497. doi: 10.1007/BF00337853. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Yura T. Induction of sigma factor synthesis in Escherichia coli by the N gene product of bacteriophage lambda. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4405–4409. doi: 10.1073/pnas.73.12.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R. N., Rogers S. G. A thermoinducible lambda phage-ColE1 plasmid chimera for the overproduction of gene products from cloned DNA segments. Gene. 1978 May;3(3):247–263. doi: 10.1016/0378-1119(78)90035-5. [DOI] [PubMed] [Google Scholar]
- Rao R. N., Rogers S. G. Plasmid pKC7: a vector containing ten restriction endonuclease sites suitable for cloning DNA segments. Gene. 1979 Sep;7(1):79–82. doi: 10.1016/0378-1119(79)90044-1. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Rowen L., Kornberg A. Primase, the dnaG protein of Escherichia coli. An enzyme which starts DNA chains. J Biol Chem. 1978 Feb 10;253(3):758–764. [PubMed] [Google Scholar]
- Scaife J. G., Heilig J. S., Rowen L., Calendar R. Gene for the RNA polymerase sigma subunit mapped in Salmonella typhimurium and Escherichia coli by cloning and deletion. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6510–6514. doi: 10.1073/pnas.76.12.6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Signer E. R., Weil J. Recombination in bacteriophage lambda. I. Mutants deficient in general recombination. J Mol Biol. 1968 Jul 14;34(2):261–271. doi: 10.1016/0022-2836(68)90251-9. [DOI] [PubMed] [Google Scholar]
- Simatake H., Rosenberg M. Purified lambda regulatory protein cII positively activates promoters for lysogenic development. Nature. 1981 Jul 9;292(5819):128–132. doi: 10.1038/292128a0. [DOI] [PubMed] [Google Scholar]
- Smiley B. L., Lupski J. R., Svec P. S., McMacken R., Godson G. N. Sequences of the Escherichia coli dnaG primase gene and regulation of its expression. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4550–4554. doi: 10.1073/pnas.79.15.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Ueda K., McMacken R., Kornberg A. dnaB protein of Escherichia coli. Purification and role in the replication of phiX174 DNA. J Biol Chem. 1978 Jan 10;253(1):261–269. [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Wickner S. H. DNA replication proteins of Escherichia coli. Annu Rev Biochem. 1978;47:1163–1191. doi: 10.1146/annurev.bi.47.070178.005503. [DOI] [PubMed] [Google Scholar]



