Abstract
Context
Type 2 diabetes in normal weight (body mass index [BMI] <25kg/m2) adults is an intriguing representation of the metabolically obese normal weight phenotype with unknown mortality consequences.
Objective
To minimize the influence of diabetes duration and voluntary weight loss on mortality, we tested the association of weight status with mortality in adults with new onset diabetes.
Design
Pooled analysis of five longitudinal cohort studies: Atherosclerosis Risk in Communities Study, 1990–2006; Cardiovascular Health Study, 1992–2008; Coronary Artery Risk Development in Young Adults, 1987–2011; Framingham Offspring Study, 1979–2007; Multi-Ethnic Study of Atherosclerosis, 2002–2011. Participants contributed 27,125 person-years of follow-up.
Setting
2,625 participants with incident diabetes
Participants
Men and women (age>40 years) who developed incident diabetes based on fasting glucose ≥ 126 mg/dL or newly-initiated diabetes medication and who had concurrent measurements of body mass index (BMI). Participants were classified as normal weight if their BMI was 18.5 to 24.99kg/m2 or overweight/obese if BMI≥25 kg/m2.
Main Outcome Measures
Total, cardiovascular, and non-cardiovascular mortality
Results
The proportion of adults who were normal weight at the time of incident diabetes ranged from 9–21% (overall=12%). Over follow-up, 449 participants died, 178 from cardiovascular causes and 253 from non-cardiovascular causes (18 were not classified). The rate of total, cardiovascular and non-cardiovascular mortality was higher in normal weight participants (248.8, 99.8, and 198.1 per 10,000 person-years, respectively) than overweight/obese participants (152.1, 67.8, and 87.9 per 10,000 person-years, respectively). Following adjustment for demographic characteristics and blood pressure, lipids, waist circumference and smoking status, hazard ratios comparing normal weight participants to overweight/obese participants for total, cardiovascular, and non-cardiovascular mortality were 2.08 (95% confidence interval [CI]: 1.52, 2.85), 1.52 (95% CI: 0.89, 2.58) and 2.32 (95% CI: 1.55, 3.48), respectively.
Conclusions
Adults who are normal weight at the time of incident diabetes have higher mortality than adults who are overweight or obese.
Keywords: type 2 diabetes, obesity, cardiovascular disease, longitudinal studies
Type 2 diabetes in normal weight adults is an intriguing and understudied representation of the metabolically obese normal weight (MONW) phenotype1 that has become increasingly common over time.2 It is not known whether the “obesity paradox” that has been observed in chronic diseases such as heart failure, chronic kidney disease and hypertension, extends to adults who are normal weight at the time of incident diabetes.3–5 . In two contemporary studies, the Translating Research Into Action for Diabetes (TRIAD) study6 and the PROactive trial7, participants with diabetes who were normal weight at the baseline examination or who lost weight during the trial (PROactive) experienced higher mortality than participants who were overweight or obese. Limitations of these prevalent disease studies are that participants had diabetes of unknown duration and participants from the PROactive trial had preexisting cardiovascular disease at baseline.
To minimize the influence of diabetes duration and unintentional or intentional weight loss secondary to diabetes development and diagnosis,8 we compared mortality between participants who were normal weight and overweight/obese at the time of incident adult-onset diabetes. We hypothesized that participants who were normal weight at the time of incident diabetes would experience higher mortality than participants who were overweight or obese.
METHODS
Study Population
Our study included 2,625 participants from the Atherosclerosis Risk in Communities (ARIC) study, Cardiovascular Health Study (CHS), Coronary Artery Risk Development in Young Adults (CARDIA) study, Framingham Offspring Study (FOS) and Multi-Ethnic Study of Atherosclerosis (MESA) who developed incident diabetes. We selected these studies because they had repeated measures of body weight, fasting glucose and medication use, a comprehensive set of commonly measured covariates and longitudinal follow-up for events and mortality.9–13 Supplementary table 1 summarizes each study's size, follow-up duration, number of examinations and examination dates.
Table 1.
Distribution of Covariates Stratified by Normal Weight Status
| ARIC | CARDIA | CHS | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Mean (SD) or No. (%) | Normal (BMI 18.5 – 24.99 kg/m2) | Overweight/ Obese (BMI ≥ 25 kg/m2) | P* | Normal (BMI 18.5 – 24.99 kg/m2) | Overweight/ Obese (BMI ≥ 25 kg/m2) | P | Normal (BMI 18.5 – 24.99 kg/m2) | Overweight/ Obese (BMI ≥ 25 kg/m2) | P |
| Participant Number (%) | 108 (8.7) | 1132 (91.3) | 28 (10.2) | 246 (89.8) | 37 (20.6) | 143 (79.4) | |||
| Age (y) | 59.8 (6.1) | 59.1 (5.9) | 0.23 | 39.4 (6.4) | 41.0 (5.8) | 0.16 | 78.6 (5.6) | 75.1 (4.7) | <0.001 |
| Non-white race (%) | 34 (31.5) | 384 (33.9) | 0.61 | 14 (50.0) | 164 (66.7) | 0.08 | 5 (13.5) | 25 (17.5) | 0.56 |
| Gender (% Female) | 51 (47.2) | 584 (51.6) | 0.38 | 14 (50.0) | 139 (56.5) | 0.51 | 20(54.1) | 79 (55.2) | 0.90 |
| Education (% < High School) | 21 (19.4) | 316 (28.0) | 0.06 | 3 (10.7) | 26 (10.6) | >.99 | 16 (43.2) | 43 (30.1) | 0.13 |
| SBP (mmHg) | 126.1 (19.3) | 128.9 (18.5) | 0.14 | 111.6 (13.3) | 121.4 (16.5) | 0.003 | 133.7 (20.3) | 134.4 (20.0) | 0.85 |
| DBP (mmHg) | 72.0 (11.1) | 73.9 (10.4) | 0.07 | 71.8 (10.8) | 79.2 (12.0) | 0.002 | 67.6 (9.9) | 70.1 (11.6) | 0.24 |
| Hypertension (%)† | 51 (47.2) | 671 (59.3) | 0.02 | 6 (21.4) | 112 (45.5) | 0.01 | 19 (51.4) | 92 (64.3) | 0.15 |
| Ever smoking (%) | 69 (63.9) | 704 (62.5) | 0.77 | 15 (55.6) | 100 (41.2) | 0.15 | 24 (68.6) | 82 (58.6) | 0.28 |
| BMI (kg/m2) | 23.2 (1.6) | 32.8 (5.7) | <.001 | 22.4 (1.8) | 37.0 (7.7) | <.001 | 22.8 (1.5) | 31.1 (4.7) | <.001 |
| Waist circ (cm) | 90.4 (9.4) | 111.1 (13.3) | <.001 | 77.6 (7.0) | 109.0 (16.7) | <.001 | 90.3 (9.1) | 108.5 (16.9) | <.001 |
| Total chol (mg/dL) | 205.5 (49.8) | 208.8 (42.3) | 0.51 | 180.4 (50.6) | 188.7 (38.9) | 0.42 | 200.0 (51.6) | 204.2 (41.8) | 0.61 |
| HDL chol (mg/dL) | 45.5 (13.2) | 42.7 (13.0) | 0.03 | 58.3 (26.8) | 42.7 (11.4) | 0.006 | 51.7 (11.2) | 47.9 (11.6) | 0.08 |
| Triglycerides (mg/dL)‡ | 126.9 (69.9) | 152.4 (104.9) | <.001 | 90.0 (83.0) | 121.5 (109.0) | 0.005 | 118.0 (122.0) | 159.0 (122.0) | 0.02 |
| LDL cholesterol (mg/dL) | 131.0 (45.2) | 130.8 (37.0) | 0.96 | 97.2 (40.3) | 114.7 (34.5) | 0.02 | 122.1 (38.0) | 122.9 (32.1) | 0.89 |
| FOS | MESA | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Normal (BMI 18.5 – 24.99 kg/m2) | Overweight/ Obese (BMI ≥ 25 kg/m2) | P* | Normal (BMI 18.5 – 24.99 kg/m2) | Overweight/ Obese (BMI ≥ 25 kg/m2) | P | |
| Mean (SD) or No. (%) | 48 (10.4) | 413 (89.6) | 72 (15.3) | 398 (84.7) | ||
| Age (y) | 59.5 (10.7) | 58.5 (9.0) | 0.47 | 68.9 (10.0) | 63.6 (9.5) | <.001 |
| Non-white race (%) | 0 (0.0) | 0 (0.0) | - | 54 (75.0) | 274 (68.8) | 0.30 |
| Gender (% Female) | 22 (45.8) | 174 (42.1) | 0.62 | 44 (61.1) | 202 (50.8) | 0.11 |
| Education (% < High School) | 5 (12.8) | 36 (10.5) | 0.59 | 22 (30.6) | 76 (19.1) | 0.03 |
| SBP (mmHg) | 129.8 (21.9) | 136.3 (18.8) | 0.03 | 123.9 (21.3) | 126.8 (19.8) | 0.26 |
| DBP (mmHg) | 76.0 (10.2) | 79.4 (12.1) | 0.06 | 68.1 (10.4) | 71.5 (10.6) | 0.01 |
| Hypertension (%)† | 25 (52.1) | 285 (69.0) | 0.02 | 43 (59.7) | 272 (68.3) | 0.15 |
| Ever smoking (%) | 38 (79.2) | 290 (70.2) | 0.20 | 31 (43.1) | 234 (59.5) | 0.009 |
| BMI (kg/m2) | 23.1 (1.7) | 32.6 (5.5) | <.001 | 23.2 (1.5) | 33.0 (5.8) | <.001 |
| Waist circ (cm) | 88.3 (10.5) | 109.7 (12.8) | <.001 | 87.5 (6.7) | 109.7 (14.2) | <.001 |
| Total chol (mg/dL) | 194.2 (46.0) | 209.8 (39.5) | 0.01 | 182.9 (43.3) | 182.6 (35.7) | 0.96 |
| HDL chol (mg/dL) | 45.6 (16.8) | 40.6 (11.9) | 0.05 | 52.7 (15.7) | 46.5 (12.4) | 0.002 |
| Triglycerides (mg/dL)‡ | 136.5 (112.0) | 181.0 (135.5) | 0.001 | 108.0 (83.0) | 131.0 (103.0) | 0.002 |
| LDL cholesterol (mg/dL) | 117.1 (37.8) | 142.1 (36.3) | 0.01 | 106.2 (33.8) | 104.8 (31.2) | 0.73 |
P value based on t-test for continuous variables and χ2 test for categorical variables
Systolic blood pressure ≥140 or diastolic blood pressure ≥90 or reported use of anti-hypertensive medications
Triglycerides are presented as median and interquartile range; statistical significance is determined using a Wilcoxin rank sum test
Institutional review boards at each of the institutions reviewed the protocols and procedures and approved the research. All participants provided written informed consent at each examination. Data were de-identified for our analysis and the Northwestern University IRB approved the research.
Diabetes and Weight Status
Diabetes was determined as fasting ( ≥ 8 hours) glucose ≥126 mg/dL (7 mmol/L)9, 11–15 or reported use of oral hypoglycemic medications or insulin. Incident diabetes was determined among participants who were free from diabetes at baseline and who met one of the above criteria at a subsequent follow-up examination.
Body mass index (BMI) was determined as the ratio of measured weight (kg) to height (in meters squared). Normal weight, overweight and obese were defined as 18.5 –24.9 kg/m2, 25–29.9 kg/m2 and ≥ 30 kg/m2, respectively.16 Participants' weight status was assigned at the examination when diabetes was identified (i.e., baseline of this analysis sample).
Follow-up Time and Mortality
Participants were followed from the examination at which diabetes was identified until they died, reached the end of their cohort surveillance or were lost to follow-up. Mortality was determined annually using cohort specific surveillance protocols and investigators adjudicated cause of death after review of all available medical records. Cardiovascular death (i.e., myocardial infarction, stroke) was adjudicated using a combination of review of death certificates for codes indicating cardiovascular disease as an underlying cause of death and proxy interviews.10–13, 17 Causes of non-cardiovascular death were not uniformly adjudicated across studies.
Covariates
Demographic characteristics, health behaviors and clinical factors available in each of the cohort studies were measured using standard protocols.9–13 We selected covariates that were commonly measured across studies. Race/ethnicity was determined according to self-report and was assessed by each component cohort study because of the known relevance of race/ethnicity to cardiovascular disease. Covariates were determined at the time of incident diabetes (i.e., baseline); however, if the measures were not available from that examination, the most recent value from a prior cohort examination was used instead.
Statistical Analysis
We compared means and standard deviations (SD) or proportions of study characteristics between normal weight and overweight/obese participants who had incident diabetes within each cohort using t-tests and χ2 tests, respectively. Kaplan-Meier survival curves with log-rank χ2 are presented to compare mortality by weight status. Because the number of participants remaining after 15 years becomes small, we truncated the presentation to 15 years of follow-up. Following confirmation of proportional hazards using log-log survival plots, we modeled the mortality hazards comparing normal weight to overweight/obese participants with diabetes (referent).
We used two strategies to generate pooled estimates: 1) cohort-specific analyses to generate effect estimates that were pooled together using fixed and random effects meta-analysis. Because effect estimates were relatively homogenous across cohorts, there were no differences between fixed and random effects and so we present fixed effects; and, 2) a pooled cohort analysis using Cox modeling with a stratification term for cohort. Because waist circumference and lipids were measured using different protocols and assays, we transformed them to z-scores in the pooled analysis. Model 1 was adjusted for age, race (non-white vs. white), sex and education (< high school vs. ≥ high school). Model 2 was adjusted for Model 1 and waist circumference, total cholesterol, high density lipoprotein cholesterol, systolic blood pressure and smoking status (current or former vs. never). Variance inflation factor and tolerance statistics indicated that the covariates in the model were not collinear.18 We tested whether sex, race, age at diabetes incidence (<65 vs. ≥65) or smoking status modified the association of weight status with mortality using multivariable Cox models with a multiplicative interaction term between each characteristic of interest and normal weight status. We determined statistical significance for the interaction based on the maximum likelihood χ2 from a nested model with and without the interaction term. Analyses were repeated for each cause of mortality.
We carried out a series of sensitivity analyses for our primary outcome of total mortality to explore alternative explanations for our findings: 1. The association between BMI per standard deviation higher and total mortality; 2. The association between waist circumference per standard deviation higher and total mortality; 3. In an attempt to reduce variability in the duration of new-onset diabetes, we restricted our analysis to participants who had elevated fasting glucose but who were not on medications to control diabetes; 4. To test whether defining diabetes using a single glucose measurement contributed to misclassification, we restricted the definition of diabetes to participants taking medications only; 5. Because Asians are more likely to develop diabetes at a lower BMI, we excluded Asians; 6. To reduce the possibility that unmeasured illness at the time of diabetes identification resulted in weight loss prior to imminent death, we excluded participants who were followed for <2 years after diabetes identification; 7. We excluded 162 participants whose BMI decreased by more than two units from the baseline examination, which may have reflected other illnesses that might predispose to death. 8. Given prior reports that overweight adults have the lowest mortality risk (particularly among older adults), we calculated mortality hazard ratios comparing normal weight and obese participants to overweight.
All analyses were carried out using Statistical Analysis Software version 10 (SAS Institute, Cary NC). Statistical significance was determined at p<0.05 (2-sided).
RESULTS
Demographic, clinical and behavioral characteristics at the time of incident diabetes are stratified by weight status in Table 1. Across cohorts, 293 (11.2%) participants had normal weight diabetes; normal weight diabetes was most common in CHS (21%) and lowest in ARIC (9%). Half (50%) of the participants were women, 36% were non-white and the mean age of participants ranged from 41 years (SD=6) in CARDIA to 76 years (SD=5) in CHS. The distribution of cardiovascular risk factors varied across cohorts.
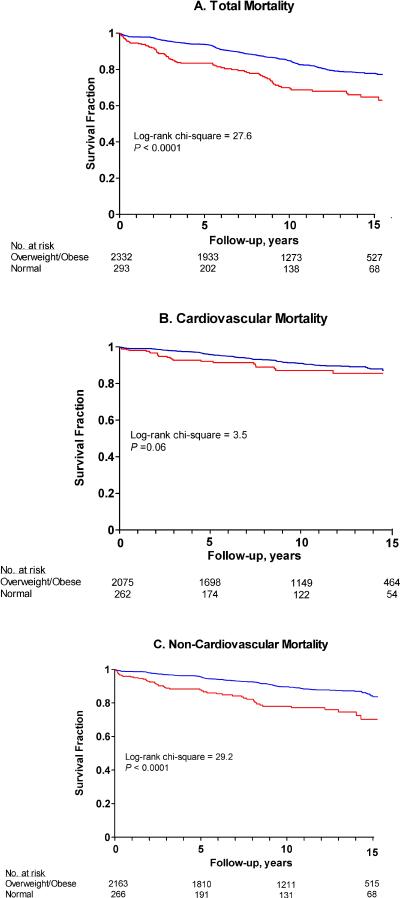
During follow-up, 449 participants died (165.5 per 10,000 person-years), 178 (6.8%) from cardiovascular causes (66.1 per 10,000 person-years) and 253 (10.4%) from non-cardiovascular causes (99.0 per 10,000 person-years); 18 causes of death were unidentified. Figure 1 displays Kaplan-Meier estimates of each type of mortality by weight status at the time of diabetes incidence. Normal weight participants experienced significantly higher total and non-cardiovascular mortality than overweight/obese participants.
Figure 1. Kaplain-Meier Survial Estimates Comparing Mortality in Participants Stratified by Weight Status at the Time of Incident Diabetes.
Red Line = Normal Weight (BMI 18.5 – 24.9 kg/2)
Blue Line=Overweight/Obese (BMI > 25 kg/m2)
Table 2 displays the crude and mulitivariable adjusted association of weight status with mortality in the pooled sample and by cohort. In the pooled sample, total, cardiovascular and non-cardiovascular mortality is higher in normal weight participants (284.8, 99.8 and 198.1 per 10,000 person-years, respectively) as compared with rates among overweight or obese participants (152.1, 67.8, 87.9 per 10,000 person-years, respectively). These patterns are consistent for total and non-cardiovascular mortality within each cohort and present for cardiovascular mortality in CHS and FOS. Mortality rates were markedly higher in CHS cohort participants who were older, on average, than other cohort participants; further, there were a relatively smaller number of participants from CHS resulting in fewer person-years of follow-up.
Table 2.
Association between weight status at the time of diabetes incidence and mortality
| Total Mortality | Cardiovascular Mortality | Non-Cardiovascular Mortality | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Weight Status at time of Diabetes Incidence | Overweight/ Obese (BMI≥ 25 kg/m2) | Normal Weight (BMI 18.5 – 24.99 kg/m2) | Overweight/ Obese (BMI≥ 25 kg/m2) | Normal Weight (BMI 18.5 – 24.99 kg/m2) | Overweight/ Obese (BMI≥25 kg/m2) | Normal Weight (BMI 18.5 – 24.99 kg/m2) |
| Full Sample | ||||||
| N | 2332 | 293 | 2086 | 265 | 2163 | 266 |
| N Events | 371 | 78 | 149 | 24 | 202 | 51 |
| Event Rate (per 10,000 P–Y) | 152.1 | 284.8 | 67.8 | 99.8 | 87.9 | 198.1 |
| Pooled analysis * | ||||||
| Unadjusted hazard ratio (95% CI) | 1 (Referent) | 1.70 (1.33, 2.18) | 1 (Referent) | 1.31 (0.85, 2.02) | 1 (Referent) | 2.03 (1.49, 2.77) |
| Multivariable Model 1† | 1 (Referent) | 1.49 (1.15, 1.93) | 1 (Referent) | 1.04 (0.65, 1.66) | 1 (Referent) | 1.79 (1.30, 2.47) |
| Multivariable Model 2‡ | 1 (Referent) | 2.08 (1.52, 2.85) | 1 (Referent) | 1.52 (0.89, 2.58) | 1 (Referent) | 2.32 (1.55, 3.48) |
| Meta-analysis § | ||||||
| Unadjusted hazard ratio (95% CI) | 1 (Referent) | 1.72 (1.33, 2.21) | 1 (Referent) | 1.24 (0.78, 1.97) | 1 (Referent) | 1.97 (1.40, 2.76) |
| Multivariable Model 1 | 1 (Referent) | 1.54 (1.18, 2.02) | 1 (Referent) | 0.98 (0.59, 1.64) | 1 (Referent) | 1.78 (1.25, 2.55) |
| Multivariable Model 2 | 1 (Referent) | 2.01 (1.44, 2.81) | 1 (Referent) | 1.29 (0.71, 2.33) | 1 (Referent) | 2.18 (1.39, 3.42) |
| Cohort-Specific | ||||||
| ARIC | ||||||
| N | 1132 | 108 | 1132 | 108 | 1060 | 102 |
| N Events | 129 | 16 | 66 | 5 | 57 | 10 |
| Event Rate (per 10,000 P–Y) | 95.6 | 121.2 | 49.2 | 38.1 | 44.3 | 78.6 |
| Unadjusted hazard ratio (95% CI) | 1 (Referent) | 1.23 (0.73, 2.07) | 1 (Referent) | 0.76 (0.31, 1.89) | 1 (Referent) | 1.73 (0.88, 3.39) |
| Multivariable Model 1 | 1 (Referent) | 1.20 (0.71, 2.02) | 1 (Referent) | 0.74 (0.30, 1.84) | 1 (Referent) | 1.68 (0.85, 3.32) |
| Multivariable Model 2 | 1 (Referent) | 1.55 (0.86, 2.79) | 1 (Referent) | 0.99 (0.37, 2.62) | 1 (Referent) | 2.10 (0.96, 4.58) |
| CARDIA | -- | -- | ||||
| N | 246 | 28 | -- | -- | 237 | 28 |
| N Events | 14 | 4 | -- | -- | 5 | 4 |
| Event Rate (per 10,000 P–Y) | 60.9 | 131.7 | -- | -- | 22.4 | 131.7 |
| Unadjusted hazard ratio (95% CI) | 1 (Referent) | 1.96 (0.64, 6.00) | -- | -- | 1 (Referent) | 5.48 (1.45, 20.71) |
| Multivariable Model 1 | 1 (Referent) | -- | -- | -- | 1 (Referent) | -- |
| Multivariable Model 2 | 1 (Referent) | -- | -- | -- | 1 (Referent) | -- |
| CHS | ||||||
| N | 143 | 37 | 143 | 37 | 101 | 27 |
| N Events | 94 | 31 | 41 | 10 | 52 | 21 |
| Event Rate (per 10,000 P–Y) | 661.6 | 1230.9 | 289.0 | 397.1 | 451.8 | 995.9 |
| Unadjusted hazard ratio (95% CI) | 1 (Referent) | 2.01 (1.33, 3.02) | 1 (Referent) | 1.42 (0.71, 2.83) | 1 (Referent) | 2.43 (1.46, 4.05) |
| Multivariable Model 1 | 1 (Referent) | 1.60 (1.05, 2.43) | 1 (Referent) | 1.04 (0.51, 2.14) | 1 (Referent) | 1.84 (1.08, 3.12) |
| Multivariable Model 2 | 1 (Referent) | 1.81 (1.08, 3.03) | 1 (Referent) | 1.26 (0.56, 2.87) | 1 (Referent) | 1.98 (1.02, 3.84) |
| FOS | ||||||
| N | 413 | 48 | 413 | 48 | 372 | 40 |
| N Events | 115 | 20 | 38 | 6 | 74 | 12 |
| Event Rate (per 10,000 P–Y) | 211.1 | 350.6 | 70.4 | 108.9 | 147.6 | 236.6 |
| Unadjusted hazard ratio (95% CI) | 1 (Referent) | 1.69 (1.05, 2.71) | 1 (Referent) | 1.56 (0.66, 3.69) | 1 (Referent) | 1.61 (0.87, 2.96) |
| Multivariable Model 1 | 1 (Referent) | 1.82 (1.04, 3.16) | 1 (Referent) | 1.36 (0.41, 4.54) | 1 (Referent) | 1.80 (0.91, 3.56) |
| Multivariable Model 2 | 1 (Referent) | 3.26 (1.47, 7.21) | 1 (Referent) | 3.45 (0.57, 20.80) | 1 (Referent) | 2.89 (1.08, 7.78) |
| MESA | ||||||
| N | 398 | 72 | -- | -- | 393 | 69 |
| N Events | 19 | 7 | -- | -- | 14 | 4 |
| Event Rate (per 10,000 P–Y) | 109.0 | 239.6 | -- | -- | 80.9 | 142.5 |
| Unadjusted hazard ratio (95% CI) | 1 (Referent) | 2.25 (0.95, 5.36) | -- | -- | 1 (Referent) | 1.80 (0.59, 5.47) |
| Multivariable Model 1 | 1 (Referent) | 1.79 (0.72, 4.41) | -- | -- | 1 (Referent) | -- |
| Multivariable Model 2 | 1 (Referent) | 3.50 (1.06, 11.61) | -- | -- | 1 (Referent) | -- |
Pooled analysis: total mortality includes all cohorts (n=2,625); cardiovascular mortality includes ARIC, CHS, FOS and MESA (n=2,351); non-cardiovascular mortality includes all cohorts (n=2,429)
Multivariable model 1 includes statistical adjustment for age, race, gender and education
Multivariable model 2 includes statistical adjustment for age, race, gender, education, waist circumference, total cholesterol, HDL-cholesterol, SBP and smoking status (ever vs. never)
Fixed effects meta-analysis for total mortality includes ARIC, CHS, FOS and MESA; cardiovascular mortality includes ARIC, CHS and FOS; non-cardiovascular mortality includes ARIC, CHS and FOS
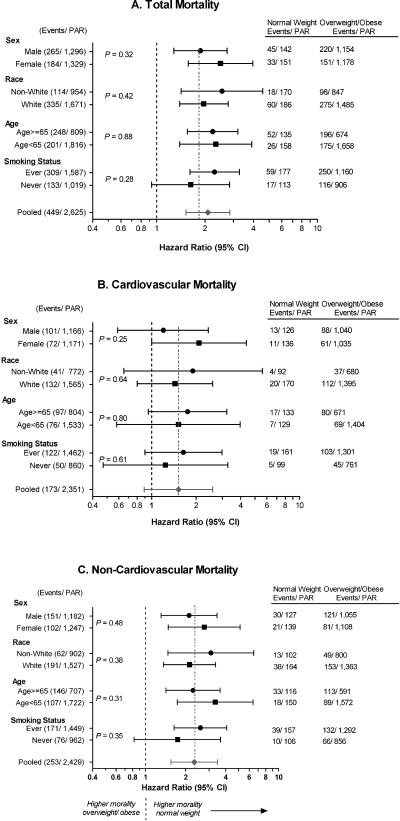
Following adjustment for covariates (model 2), participants with normal weight diabetes experienced a significantly elevated total mortality (hazard ratio [HR]=2.08, 95% CI: 1.52, 2.85) and non-cardiovascular mortality (HR=2.32, 95% CI: 1.55, 3.48). Although the hazard for cardiovascular mortality was elevated, the association was not statistically significant (HR=1.52, 95% CI: 0.89, 2.58). Results generated using meta-analysis demonstrated similar effect estimates. Findings were consistent across cohorts, though not always statistically significant. Participants with normal weight diabetes had higher mortality from all causes than overweight/obese participants across strata of gender, age, race and smoking (Figure 2).
Figure 2. Adjusted hazard ratios (95% confidence intervals) of mortality by weight status (normal weight vs. overweight/obese), stratified by subgroup.
Adjusted for age, race, gender, education, waist circumference, total cholesterol, HDL-cholesterol, systolic blood pressure, smoking status (ever. vs. never).
Statistical significance (P-value) for interaction term based on the maximum likelihood χ2 from a proportional hazards model that included a multiplicative interaction term.
PAR= Population at Risk. Normal weight = BMI 18.5 – 24.99 kg/m2; Overweight/Obese = BMI >= 25 kg/m2.
The findings from each of our sensitivity analyses are presented in Table 3. BMI (per standard deviation higher) was not associated with total mortality but waist circumference was significantly positively associated with mortality Normal weight status was positively associated with mortality in each of the additional analyses. When we stratified weight at the time of diabetes into three levels we observed higher total mortality in normal weight as compared with overweight (referent) participants whereas mortality hazards did not differ between obese vs. overweight.
Table 3.
Association of BMI and Weight Status with Total Mortality in the Pooled Cohort: Results of Sensitivity Analyses
| N | N Events | Event Rate | Unadjusted | Multivariable Model 1 * | Multivariable Model 2 † | |
|---|---|---|---|---|---|---|
| 1. BMI (per SD) | 2625 | 449 | 165.5 | 0.90 (0.81, 1.00) | 1.04 (0.93, 1.17) | 1.00 (0.88, 1.13) |
| 2. Waist Circumference (per SD) | 2625 | 449 | 165.5 | 1.08 (0.97, 1.19) | 1.18 (1.06, 1.31) | 1.14 (1.02, 1.28) |
| 3. Diagnosis by fasting glucose only ‡ | ||||||
| Overweight/ Obese (BMI ≥ 25 kg/m2) | 1657 | 193 | 110.3 | 1 (Referent) | 1 (Referent) | 1 (Referent) |
| Normal weight (BMI 18.5 – 24.9 kg/m2) | 181 | 35 | 190.5 | 1.60 (1.12, 2.30) | 1.38 (0.95, 2.00) | 2.20 (1.43, 3.38) |
| 4. Diagnosis by medication alone ‡ | ||||||
| Overweight/Obese (BMI ≥ 25 kg/m2) | 1,344 | 204 | 164.4 | 1 (Referent) | 1 (Referent) | 1 (Referent) |
| Normal weight (BMI 18.5 – 24.9 kg/m2) | 161 | 30 | 222.8 | 1.32 (0.89, 1.94) | 1.38 (0.91, 2.08) | 1.96 (1.16, 3.31) |
| 5. Excluding Asians | ||||||
| Overweight/ Obese (BMI ≥ 25 kg/m2) | 2,307 | 368 | 138.4 | 1 (Referent) | 1 (Referent) | 1 (Referent) |
| Normal weight (BMI 18.5 – 24.9 kg/m2) | 268 | 77 | 291.4 | 1.73 (1.35, 2.21) | 1.47 (1.14, 1.91) | 2.06 (1.50, 2.83) |
| 6. Follow-up for < 2 years | ||||||
| Overweight/ Obese (BMI ≥ 25 kg/m2) | 2,285 | 337 | 138.4 | 1 (Referent) | 1 (Referent) | 1 (Referent) |
| Normal weight (BMI 18.5 – 24.9 kg/m2) | 279 | 67 | 246.2 | 1.65 (1.27, 2.15) | 1.46 (1.11, 1.92) | 2.05 (1.46, 2.87) |
| 7. BMI decreased by < 2 units from baseline | ||||||
| Overweight/ obese (BMI ≥ 25 kg/m2) | 2,217 | 344 | 147.1 | 1 (Referent) | 1 (Referent) | 1 (Referent) |
| Normal weight (BMI 18.5 – 24.9 kg/m2) | 245 | 60 | 261.1 | 1.66 (1.26, 2.19) | 1.48 (1.11, 1.97) | 2.07 (1.47, 2.92) |
| 8. Weight status | ||||||
| Normal weight (BMI 18.5 – 24.9 kg/m2) | 293 | 78 | 284.8 | 1.68 (1.28, 2.20) | 1.65 (1.24, 2.19) | 2.02 (1.47, 2.77) |
| Overweight (BMI 25.0 – 29.9 kg/m2) | 858 | 163 | 174.9 | 1 (Referent) | 1 (Referent) | 1 (Referent) |
| Obese (BMI ≥ 30 kg/m2) | 1,474 | 208 | 138.0 | 0.97 (0.79, 1.20) | 1.22 (0.98, 1.52) | 0.86 (0.64, 1.16) |
Multivariable model 1 includes statistical adjustment for age, race, gender and education
Multivariable model 2 includes statistical adjustment for age, race, gender, education, waist circumference, total cholesterol, HDL-cholesterol, SBP and smoking status (ever vs. never)
Includes ARIC, CARDIA, FOS and MESA
DISCUSSION
In our pooled longitudinal study, participants who were normal weight at the time of incident diabetes experienced higher total and non-cardiovascular mortality as compared with those who were overweight or obese. Cardiovascular mortality was non-significantly elevated in participants who were normal weight as compared with those who were overweight or obese. Findings were consistent across demographic categories and smoking status and persisted following adjustment for known cardiovascular disease risk factors.
It was unexpected that weight status was not associated with cardiovascular mortality. However, crude cardiovascular mortality rates were higher in normal weight vs. overweight/obese participants and hazard ratios from fully adjusted models reflect elevated mortality. Consequently, we interpreted the absence of statistical significance as a byproduct of low statistical power due to the relatively smaller number of cardiovascular events.
Overweight and obese patients with end stage renal disease have better health outcomes than leaner patients.19–21 Similarly, lean hypertensives (the cutpoint for “lean” varies across studies)22 and persons with heart failure3 have worse health outcomes than their heavier counterparts. Even among persons without known chronic diseases, heavier weight may only be positively associated with long-term (>15 years) mortality.23 Our findings are consistent with the existing literature in other prevalent disease cohorts, including those of persons with diabetes.6, 7, 24, 25
Lower body weight in the presence of obesity-related metabolic disorders may reflect underlying illness that predisposes to mortality. Prior research has attempted to reduce the influence of latent illness by excluding those who died early (2–5 years) during the follow-up period. We did not have an adequate number of events over an extended follow-up period (> 15 years) to study long-term mortality,23 and so our findings could reflect higher mortality among persons who were already ill for reasons unrelated to diabetes. Statistical adjustment for demographic characteristics (e.g., socioeconomic status) and health behaviors (e.g., smoking) associated with other causes of mortality, did not change our findings. Despite having a leaner body habitus, cigarette smokers are more insulin resistant26, more likely to develop diabetes,27 and have increased mortality as compared with non-smokers. However, we report that the elevated mortality in normal weight participants is not entirely attributable to higher smoking as findings are similar among smokers and non-smokers.
The primary features distinguishing our study from the contemporary PROactive trial7 and the TRIAD studies6 (as well as earlier studies addressing this question24, 25) are that we 1) defined weight status at the time of incident diabetes; and, 2) identified an elevated risk of mortality in normal weight adults who did not have comorbid cardiovascular diseases (e.g., coronary heart disease, cerebrovascular disease). Although unexplained or unintentional weight loss, despite hunger and regular eating is most commonly described as a symptom of type 1 diabetes, it is often present in type 2 diabetes.8 Intentional weight loss is recommended following the identification of type 2 diabetes based on findings that adults who lose weight have better glycemic control and other cardiovascular disease risk factors.28 Both of these scenarios could confound the ability to describe the association between weight status and mortality if weight status is determined at the time of prevalent diabetes.
Latent Autoimmune Diabetes in Adults (LADA)29 is phenotypically similar to type 1 diabetes because of apparent β cell destruction and presentation in normal weight adults. Some normal weight adults with diabetes may have LADA, but it is not possible identify LADA without measuring autoantibodies such as GAD or C-peptide—neither of which were universally measured in these cohort studies. We did not have access to the type of diabetes control medication (oral hypoglycemic vs. insulin replacement) across all cohort studies in our analysis. Consequently, we are unable to determine whether participants who were normal weight at the time of diabetes incidence in our study have LADA. Despite this limitation, our findings suggest that regardless of diabetes type, normal weight status at the time of diabetes incidence may be a straightforward marker to identify elevated mortality risk.
In our epidemiologic study, normal weight is determined based on BMI and not on a direct measure of adiposity. Higher BMI could be the result of more lean muscle mass, which is more insulin sensitive than adipose tissue and, consequently, metabolically favorable. If, as suggested,30–32 insulin resistance is the primary underlying factor in cardiovascular disease, then unmeasured fat mass and insulin sensitivity may be a significant source of residual confounding among normal weight adults. Waist circumference was directly positively associated with mortality in our sample and the strength of association between normal weight status and total mortality became modestly stronger when waist circumference was included in our models. Our adjusted findings may reflect an adverse role of lower lean mass on mortality in participants who are normal weight at the time of incident diabetes. Because our initial hypothesis was for a threshold effect of BMI in the normal weight category, it was not unexpected that when BMI was studied continuously in relation to mortality that the effect we hypothesized was obscured and that there was no association.
Age-related loss of lean muscle mass and bone (i.e., sarcopenia) could result in a lower body weight despite greater fat mass in older adults. Older adults who are “frail” have elevated mortality from all causes.33 Although we did not directly assess frailty, we excluded underweight participants from our analyses, tested for interaction by age, excluded participants who died within two years of inception into the cohort and participants who lost weight. In each of these sensitivity analyses, normal weight status remained associated with higher mortality and there was no interaction by age. While the effect estimates for cardiovascular mortality in older adults included the null, our tests for statistical interaction indicate that there is no difference between strata.
Leaner adults with diabetes may have been screened less rigorously for diabetes and its complications by their healthcare providers. Consequently, cardiovascular disease risk factors may have gone untreated or under-treated. One strength of having carried out our investigation in a cohort study vs. a health practice plan is that all participants were examined at regular intervals independent of healthcare complaints and weight status. By including assessments of cardiovascular disease risk factors in our multivariable models, we were able to statistically adjust for the presence of other cardiovascular risk factors at the time of diabetes identification that could have precipitated mortality.
Strengths and Limitations
A cohort comprised of adults with incident disease (an inception cohort) is the strongest design to investigate our question because the likelihood of developing complications is positively associated with diabetes duration and because participants may have initiated weight loss because of their diagnosis. While participants could have developed diabetes in between study intervals, the length between exams across studies ranged from 2 to 5 years and variability in diabetes duration at baseline is truncated. Sensitivity analyses excluding participants using medications confirmed our findings. The robustness of our findings are reflected in the consistent associations within each cohort and in subgroups defined by age, race, sex and smoking status.
Smoking status is a potentially important modifier of the association and our ability to distinguish smoking burden (e.g., duration, timing and amount) was hindered by the inconsistent methods of capturing smoking across cohorts. As a result, we could only crudely stratify to compare participants who ever (comprised of current and former) reported smoking to those who never smoked. Because these cardiovascular disease cohort studies did not commonly validate non-cardiovascular causes of morbidity or mortality, and so we were unable to determine the specific causes of elevated non-cardiovascular mortality or of medical conditions that could promote the onset of diabetes in normal weight adults. Similarly, we could not study the contributions of medications for other illnesses that are associated with higher mortality and that could promote the onset of diabetes (e.g., antidepressants). Despite our attempts to rule out illness through our sensitivity analyses, it is possible that participants who were normal weight at the time of diabetes incidence may have had underlying non-cardiovascular illnesses predisposing them to mortality.
Conclusion
Mechanisms to explain our findings of higher mortality in adults who are normal weight at the time of incident diabetes are unknown. However, previous research suggests that normal weight persons with diabetes have a different genetic profile than overweight or obese persons with diabetes.34 If those same genetic variants that predispose to diabetes are associated with other illnesses, these individuals may be “genetically loaded” towards experiencing higher mortality. Future research in normal weight persons with diabetes should test these genetic hypotheses, along with other plausible mechanisms to account for higher mortality including inflammation, the distribution and action of adipose tissue, atherosclerosis burden and the composition of fatty plaques, and pancreatic β-cell function. In summary, findings from our observational study that adults who are normal weight at the time of diabetes incidence experienced higher mortality than overweight or obese adults with diabetes are relevant to growing segments of our population including older adults and non-whites (e.g., Asian35, black36) who are more likely to experience normal weight diabetes.
Acknowledgements
The Principal Investigator, Dr. Mercedes Carnethon, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This research is funded by the National Institute of Diabetes, Digestive and Kidney Disease grant number R21DK082903. The funding agency did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review or approval of the manuscript.
Mr. Peter de Chavez carried out all statistical analyses in the study under the direction of the Principal Investigator.
MESA was supported by contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org
The Atherosclerosis Risk in Communities study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. The authors thank the staff and participants of the ARIC study for their important contributions.
CARDIA is supported by grant 5 R01 HL078972 from the National Heart Lung and Blood Institute (NHLBI) and was partially supported by contracts N01 – HC-48047, N01-HC-48048, N01-HC-48049, N01-HC-48050 and N01-HC-95095 from the National Heart, Lung, and Blood Institute/National Institutes of Health. The authors gratefully acknowledge the CARDIA Study participants and staff for their valuable contributions.
CHS: The research reported in this article was supported by contracts N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
References
- 1.Ruderman NB, Schneider SH, Berchtold P. The “metabolically-obese,” normal-weight individual. Am J Clin Nutr. 1981;34(8):1617–1621. doi: 10.1093/ajcn/34.8.1617. [DOI] [PubMed] [Google Scholar]
- 2.Gregg EW, Cadwell BL, Cheng YJ, et al. Trends in the prevalence and ratio of diagnosed to undiagnosed diabetes according to obesity levels in the U.S. Diabetes Care. 2004;27(12):2806–2812. doi: 10.2337/diacare.27.12.2806. [DOI] [PubMed] [Google Scholar]
- 3.Lavie CJ, Milani RV, Ventura HO, Romero-Corral A. Body composition and heart failure prevalence and prognosis: getting to the fat of the matter in the “obesity paradox”. Mayo Clin Proc. 2010;85(7):605–608. doi: 10.4065/mcp.2010.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uretsky S, Messerli FH, Bangalore S, et al. Obesity paradox in patients with hypertension and coronary artery disease. Am J Med. 2007;120(10):863–870. doi: 10.1016/j.amjmed.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt D, Salahudeen A. The Obesity-Survival Paradox in Hemodialysis Patients: Why Do Overweight Hemodialysis Patients Live Longer? Nutrition in Clinical Practice. 2007;22(1):11–15. doi: 10.1177/011542650702200111. [DOI] [PubMed] [Google Scholar]
- 6.McEwen LN, Kim C, Karter AJ, et al. Risk Factors for Mortality Among Patients With Diabetes. Diabetes Care. 2007;30(7):1736–1741. doi: 10.2337/dc07-0305. [DOI] [PubMed] [Google Scholar]
- 7.Doehner W, Erdmann E, Cairns R, et al. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co-morbidity: An analysis of the PROactive study population. Int J Cardiol. 2011 doi: 10.1016/j.ijcard.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2012;35(Supplement 1):S64–S71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. Journal of Clinical Epidemiology. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 10.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 11.ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) Study: Design and Objectives. American Journal of Epidemiology. 1989;129(4):687–699. [PubMed] [Google Scholar]
- 12.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 13.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4(4):518–525. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 14.Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. Jama. 2000;283(17):2253–2259. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 15.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41(2):264–270. [PubMed] [Google Scholar]
- 16.anonymous Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Archives of Internal Medicine. 1998;158(17):1855–1867. doi: 10.1001/archinte.158.17.1855. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet. 1999;353(9147):89–92. doi: 10.1016/S0140-6736(98)10279-9. [DOI] [PubMed] [Google Scholar]
- 18.Kleinbaum DG, Kupper LL, Nizam A, Muller KE. Applied Regression Analyses and Other Multivariable Methods. 4th Edition ed Thomson Brooks/Cole; Belmont, CA: 2008. [Google Scholar]
- 19.Kopple JD, Zhu X, Lew NL, Lowrie EG. Body weight-for-height relationships predict mortality in maintenance hemodialysis patients. Kidney Int. 1999;56(3):1136–1148. doi: 10.1046/j.1523-1755.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 20.Leavey SF, McCullough K, Hecking E, Goodkin D, Port FK, Young EW. Body mass index and mortality in `healthier' as compared with `sicker' haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2001;16(12):2386–2394. doi: 10.1093/ndt/16.12.2386. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe RA, Ashby VB, Daugirdas JT, Agodoa LY, Jones CA, Port FK. Body size, dose of hemodialysis, and mortality. Am J Kidney Dis. 2000;35(1):80–88. doi: 10.1016/S0272-6386(00)70305-2. [DOI] [PubMed] [Google Scholar]
- 22.Uretsky S, Messerli FH, Bangalore S, et al. Obesity Paradox in Patients with Hypertension and Coronary Artery Disease. The American Journal of Medicine. 2007;120(10):863–870. doi: 10.1016/j.amjmed.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Dyer AR, Stamler J, Garside DB, Greenland P. Long-term consequences of body mass index for cardiovascular mortality: the Chicago Heart Association Detection Project in Industry study. Ann Epidemiol. 2004;14(2):101–108. doi: 10.1016/S1047-2797(03)00121-2. [DOI] [PubMed] [Google Scholar]
- 24.Klein R, Klein BE, Moss SE. Is obesity related to microvascular and macrovascular complications in diabetes? The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Intern Med. 1997;157(6):650–656. [PubMed] [Google Scholar]
- 25.Ross C, Langer RD, Barrett-Connor E. Given diabetes, is fat better than thin? Diabetes Care. 1997;20(4):650–652. doi: 10.2337/diacare.20.4.650. [DOI] [PubMed] [Google Scholar]
- 26.Attvall S, Fowelin J, Lager I, Von Schenck H, Smith U. Smoking induces insulin resistance--a potential link with the insulin resistance syndrome. Journal of Internal Medicine. 1993;233(4):327–332. doi: 10.1111/j.1365-2796.1993.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 27.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. Jama. 2007;298(22):2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 28.Standards of Medical Care in Diabetes—2012. Diabetes Care. 2012;35(Supplement 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naik RG, Brooks-Worrell BM, Palmer JP. Latent Autoimmune Diabetes in Adults. Journal of Clinical Endocrinology & Metabolism. 2009;94(12):4635–4644. doi: 10.1210/jc.2009-1120. [DOI] [PubMed] [Google Scholar]
- 30.Folsom AR, Rasmussen ML, Chambless LE, et al. Prospective associations of fasting insulin, body fat distribution, and diabetes with risk of ischemic stroke. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Diabetes Care. 1999;22(7):1077–1083. doi: 10.2337/diacare.22.7.1077. [DOI] [PubMed] [Google Scholar]
- 31.Folsom AR, Szklo M, Stevens J, Liao F, Smith R, Eckfeldt JH. A prospective study of coronary heart disease in relation to fasting insulin, glucose, and diabetes. The Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care. 1997;20(6):935–942. doi: 10.2337/diacare.20.6.935. [DOI] [PubMed] [Google Scholar]
- 32.Ruige JB, Assendelft WJJ, Dekker JM, Kostense PJ, Heine RJ, Bouter LM. Insulin and Risk of Cardiovascular Disease : A Meta-Analysis. Circulation. 1998;97(10):996–1001. doi: 10.1161/01.cir.97.10.996. [DOI] [PubMed] [Google Scholar]
- 33.Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: A systematic literature review. Ageing Res Rev. 2012 doi: 10.1016/j.arr.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Perry JRB, Voight BF, Yengo L, et al. Stratifying Type 2 Diabetes Cases by BMI Identifies Genetic Risk Variants in LAMA1 and Enrichment for Risk Variants in Lean Compared to Obese Cases. PLoS Genet. 2012;8(5):e1002741. doi: 10.1371/journal.pgen.1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abate N, Chandalia M. Ethnicity and type 2 diabetes: focus on Asian Indians. J Diabetes Complications. 2001;15(6):320–327. doi: 10.1016/s1056-8727(01)00161-1. [DOI] [PubMed] [Google Scholar]
- 36.Carnethon MR, Palaniappan LP, Burchfiel CM, Brancati FL, Fortmann SP. Serum Insulin, Obesity, and the Incidence of Type 2 Diabetes in Black and White Adults: The Atherosclerosis Risk in Communities Study: 1987–1998. Diabetes Care. 2002;25(8):1358–1364. doi: 10.2337/diacare.25.8.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]