Abstract
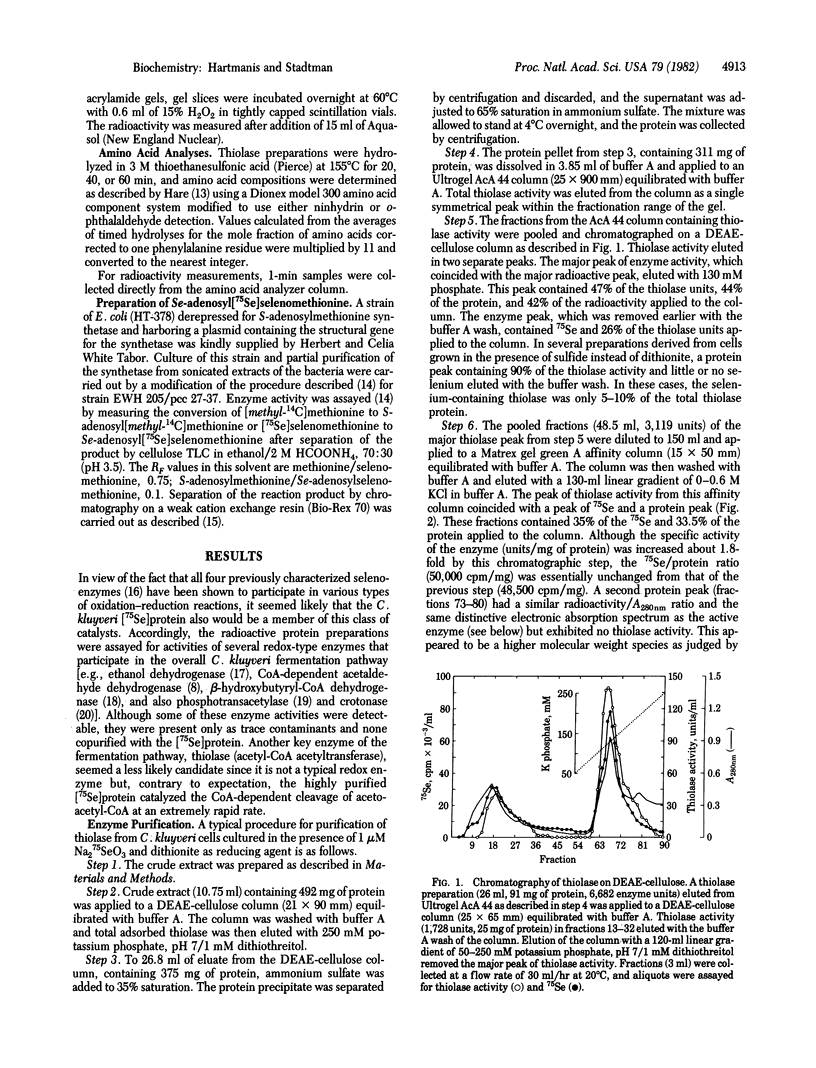
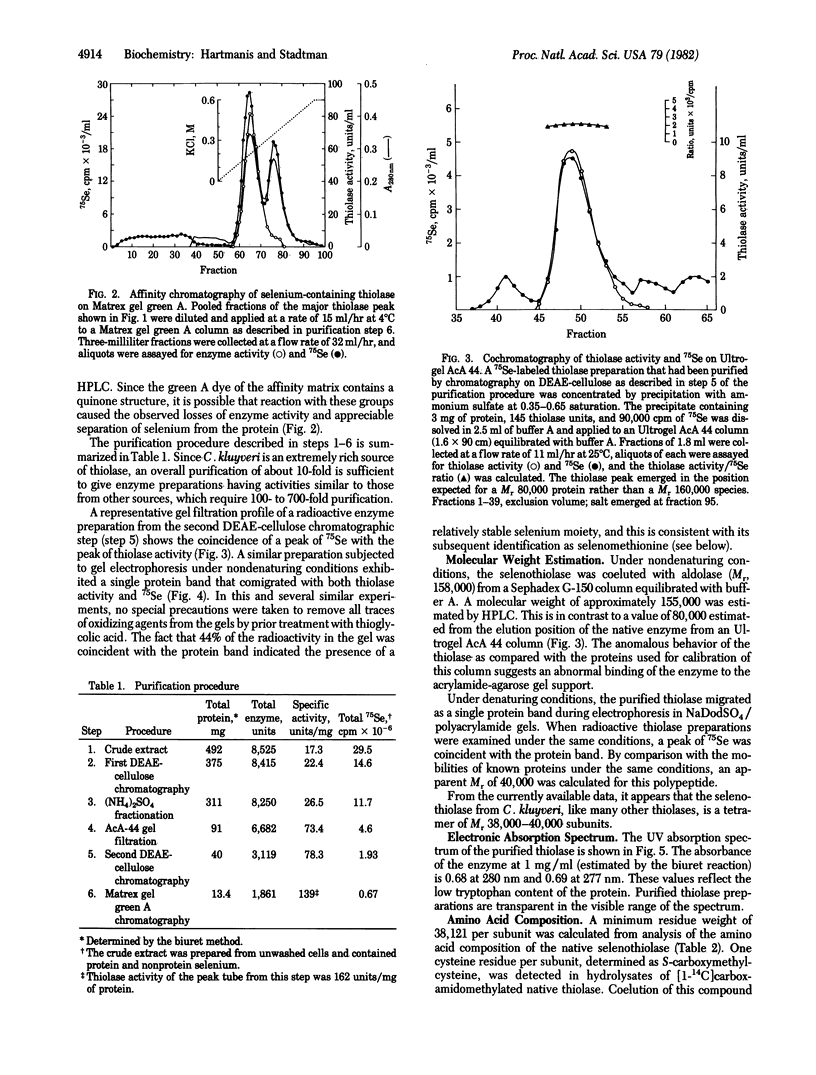
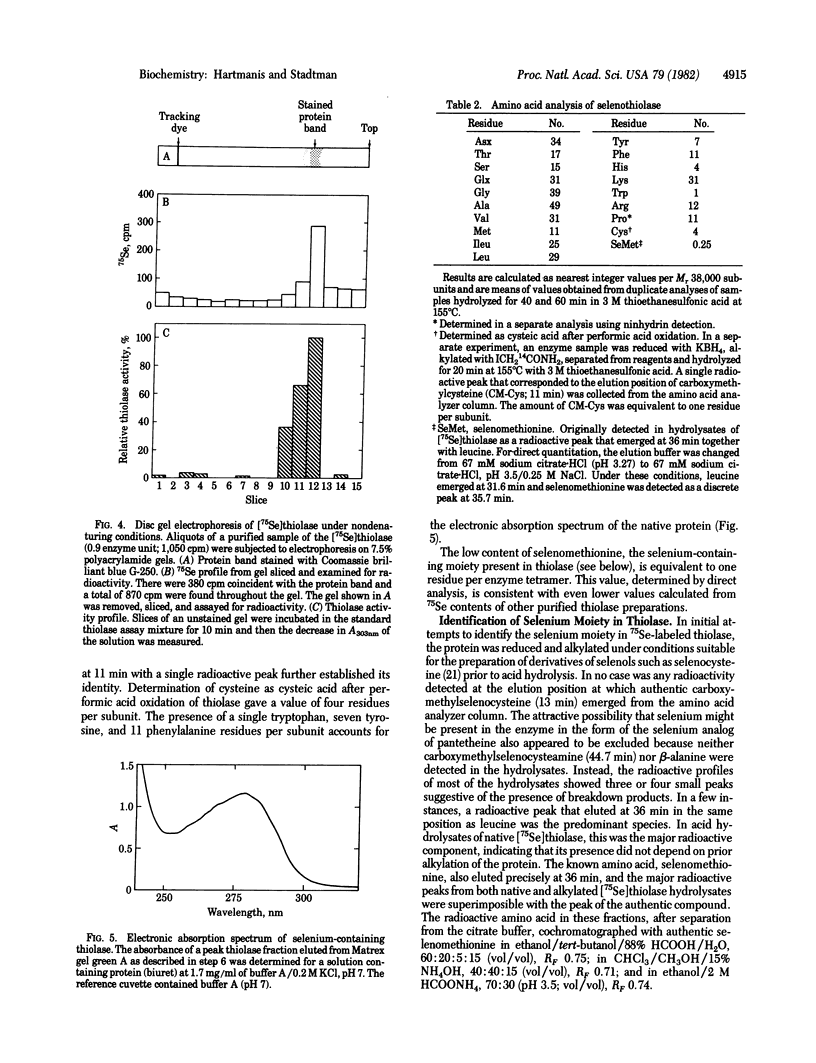
Clostridium kluyveri grown in the presence of 1 muM Na2(75)SeO3 produces a thiolase that copurifies with 75Se. Based on several criteria, the selenium moiety in this protein is selenomethionine. The 75Se-labeled amino acid in acid hydrolysates of the radioactive protein cochromatographed with authentic selenomethionine on an amino acid analyzer and on TLC plates in acidic and basic solvents. Incubation with S-adenosylmethionine synthetase and ATP converted the 75Se-labeled amino acid to a radioactive basic product that was indistinguishable from authentic Se-adenosylselenomethionine by ion exchange and TLC. The native selenoenzyme, Mr 155,000-158,000, is composed of four subunits of Mr 38,000-40,000. Thiolase of similar molecular weight that is less acidic and lacks selenium is also produced by C. kluyveri. The factors that control the relative levels of the two enzymes in the cell have not been identified.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berndt H., Schlegel H. G. Kinetics and properties of beta-ketothiolase from Clostridium pasteurianum. Arch Microbiol. 1975 Mar 12;103(1):21–30. doi: 10.1007/BF00436325. [DOI] [PubMed] [Google Scholar]
- Chen C. S., Stadtman T. C. Selenium-containing tRNAs from Clostridium sticklandii: cochromatography of one species with L-prolyl-tRNA. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1403–1407. doi: 10.1073/pnas.77.3.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone J. E., Del Río R. M., Davis J. N., Stadtman T. C. Chemical characterization of the selenoprotein component of clostridial glycine reductase: identification of selenocysteine as the organoselenium moiety. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2659–2663. doi: 10.1073/pnas.73.8.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Duncombe G. R., Frerman F. E. Molecular and catalytic properties of the acetoacetyl-coenzyme A thiolase of Escherichia coli. Arch Biochem Biophys. 1976 Sep;176(1):159–170. doi: 10.1016/0003-9861(76)90152-1. [DOI] [PubMed] [Google Scholar]
- Feigenbaum J., Schulz H. Thiolases of Escherichia coli: purification and chain length specificities. J Bacteriol. 1975 May;122(2):407–411. doi: 10.1128/jb.122.2.407-411.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring U., Riepertinger C. Dissoziation und Rekonstitution der Thiolase. Eur J Biochem. 1968 Nov;6(2):281–292. doi: 10.1111/j.1432-1033.1968.tb00447.x. [DOI] [PubMed] [Google Scholar]
- Gehring U., Riepertinger C., Lynen F. Reinigung und Kristallisation der Thiolase, Untersuchungen zum Wirkungsmechanismus. Eur J Biochem. 1968 Nov;6(2):264–280. doi: 10.1111/j.1432-1033.1968.tb00446.x. [DOI] [PubMed] [Google Scholar]
- Hare P. E. Subnanomole-range amino acid analysis. Methods Enzymol. 1977;47:3–18. doi: 10.1016/0076-6879(77)47003-4. [DOI] [PubMed] [Google Scholar]
- Kornblatt J. A., Rudney H. Two forms of acetoacetyl coenzyme A thiolase in yeast. I. Separation and properties. J Biol Chem. 1971 Jul 25;246(14):4417–4423. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MUDD S. H., CANTONI G. L. Selenomethionine in enzymatic transmethylations. Nature. 1957 Nov 16;180(4594):1052–1052. doi: 10.1038/1801052a0. [DOI] [PubMed] [Google Scholar]
- Madan V. K., Hillmer P., Gottschalk G. Purification and properties of NADP-dependent L(+)-3-hydroxybutyryl-CoA dehydrogenase from Clostridium kluyveri. Eur J Biochem. 1973 Jan 3;32(1):51–56. doi: 10.1111/j.1432-1033.1973.tb02577.x. [DOI] [PubMed] [Google Scholar]
- Markham G. D., Hafner E. W., Tabor C. W., Tabor H. S-Adenosylmethionine synthetase from Escherichia coli. J Biol Chem. 1980 Oct 10;255(19):9082–9092. [PubMed] [Google Scholar]
- Mazzei Y., Negrel R., Ailhaud G. Purification and some properties of thiolase from Escherichia coli. Biochim Biophys Acta. 1970 Oct 14;220(1):129–131. doi: 10.1016/0005-2744(70)90238-x. [DOI] [PubMed] [Google Scholar]
- Middleton B. The oxoacyl-coenzyme A thiolases of animal tissues. Biochem J. 1973 Apr;132(4):717–730. doi: 10.1042/bj1320717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STERN J. R. Optical properties of aceto-acetyl-S-coenzyme A and its metal chelates. J Biol Chem. 1956 Jul;221(1):33–44. [PubMed] [Google Scholar]
- Schwabe D., Huth W. Immunochemical aspects, molecular and kinetic properties of multiple forms of acetyl-CoA acetyltransferase from rat liver mitochondria. Biochim Biophys Acta. 1979 Oct 26;575(1):112–120. doi: 10.1016/0005-2760(79)90136-x. [DOI] [PubMed] [Google Scholar]
- Staack H., Binstock J. F., Schulz H. Purification and properties of a pig heart thiolase with broad chain length specificity and comparison of thiolases from pig heart and Escherichia coli. J Biol Chem. 1978 Mar 25;253(6):1827–1831. [PubMed] [Google Scholar]
- Stadtman T. C. Selenium-dependent enzymes. Annu Rev Biochem. 1980;49:93–110. doi: 10.1146/annurev.bi.49.070180.000521. [DOI] [PubMed] [Google Scholar]



