Abstract
Acupuncture is an invasive procedure commonly used to relieve pain. Acupuncture is practiced worldwide, despite difficulties in reconciling its principles with evidence-based medicine. We found that adenosine, a neuromodulator with anti-nociceptive properties, was released during acupuncture in mice and that its anti-nociceptive actions required adenosine A1 receptor expression. Direct injection of an adenosine A1 receptor agonist replicated the analgesic effect of acupuncture. Inhibition of enzymes involved in adenosine degradation potentiated the acupuncture-elicited increase in adenosine, as well as its anti-nociceptive effect. These observations indicate that adenosine mediates the effects of acupuncture and that interfering with adenosine metabolism may prolong the clinical benefit of acupuncture.
Acupuncture is a procedure in which fine needles are inserted into an individual at discrete points and then manipulated, with the intent of relieving pain. Since its development in China around 2,000 B.C., acupuncture has become worldwide in its practice1. Although Western medicine has treated acupuncture with considerable skepticism2, a broader worldwide population has granted it acceptance. For instance, the World Health Organization endorses acupuncture for at least two dozen conditions3 and the US National Institutes of Health issued a consensus statement proposing acupuncture as a therapeutic intervention for complementary medicine. Perhaps most tellingly, the U.S. Internal Revenue Service approved acupuncture as a deductible medical expense in 1973.
Although the analgesic effect of acupuncture is well documented, little is understood about its biological basis. Insertion of the acupuncture needles in itself is not sufficient to relieve pain4. An acupuncture session typically lasts for 30 min, during which the needles are intermittently rotated, electrically stimulated or, in some cases, heated. The pain threshold is reported to slowly increase and to outlast the treatment4. The primary mechanism implicated in the anti-nociceptive effect of acupuncture involves release of opioid peptides in the CNS in response to the long-lasting activation of ascending sensory tracks during the intermittent stimulation4–6. However, a centrally acting agent cannot explain why acupuncture is conventionally applied in close proximity to the locus of pain and why the analgesic effects of acupuncture are restricted to the ipsilateral side7,8.
RESULTS
Acupuncture triggers adenosine and ATP metabolites release
ATP is released in response to either mechanical and electrical stimulation or heat. Once released, ATP acts as a transmitter that binds to purinergic receptors, including P2X and P2Y receptors9,10. ATP cannot be transported back into the cell but is rapidly degraded to adenosine by several ectonucleotidases before re-uptake10. Thus, adenosine acts as an analgesic agent that suppresses pain through Gi-coupled A1-adenosine receptors11–13. To determine whether adenosine is involved in the anti-nociceptive effects of acupuncture, we first asked whether the extracellular concentration of adenosine increases during acupuncture.
We collected samples of interstitial fluid by a microdialysis probe implanted in the tibialis anterior muscle/subcutis of adult mice at a distance of 0.4–0.6 mm from the `Zusanli point', which is located 3–4 mm below and 1–2 mm lateral for the midline of the knee4. Adenine nucleotides and adenosine were quantified using high-performance liquid chromatography (HPLC) with ultraviolet absorbance before, during and after acupuncture (Fig. 1a)14,15. At baseline, the concentrations of ATP, ADP, AMP and adenosine were in the low nanomolar range (Fig. 1b), consistent with previous reports16,17. Acupuncture applied by gentle manual rotation of the acupuncture needle every 5 min for a total of 30 min sharply increased the extracellular concentrations of all purines detected (Fig. 1b). Adenosine concentration increased ~24-fold (253.5 ± 81.1 nM from a baseline of 10.6 ± 6.7 nM) during the 30-min acupuncture session (Fig. 1c). The extracellular concentration of ATP returned to baseline after acupuncture, whereas adenosine, AMP and ADP remained significantly elevated (adenosine and AMP, P < 0.01; ADP, P < 0.05, paired t test compared to 0 min) at 60 min (Fig. 1c). Notably, previous studies have shown that deep brain stimulation is also associated with a severalfold increase in extracellular ATP and adenosine. Similar to electroacupuncture and transcutaneous electrical nerve stimulation, deep brain stimulation delivers electrical stimulation that triggers an increase in extracellular adenosine concentration18.
Figure 1.
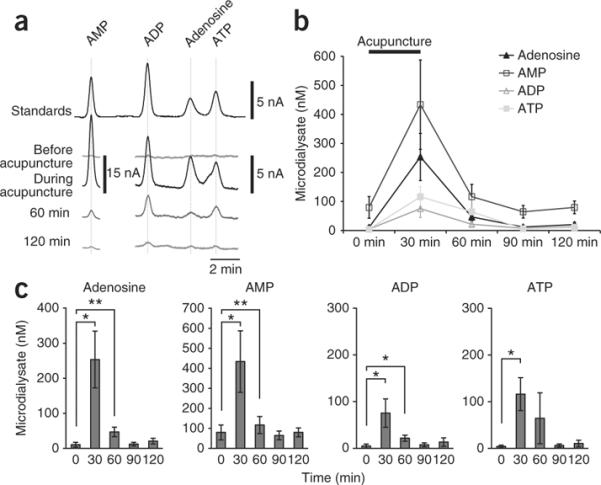
Acupuncture triggers an increase in the extracellular concentration of ATP, ADP, AMP and adenosine. (a) Representative HPLC chromatograms before, during and after acupuncture. The samples were collected by a microdialysis probe implanted in close proximity to the Zusanli point. Standards of adenosine, AMP, ADP and ATP (0.3 μM each) are shown on top. (b) Time course of purine release in response to acupuncture. (c) Histogram summarizing the mean concentrations of adenosine, AMP, ADP and ATP during baseline nonstimulated conditions, as well as during and following acupuncture (*P < 0.05, **P < 0.01, paired t test compared to 0 min, n = 8). Error bars indicate s.e.m.
Effect of local application of A1 receptor agonist
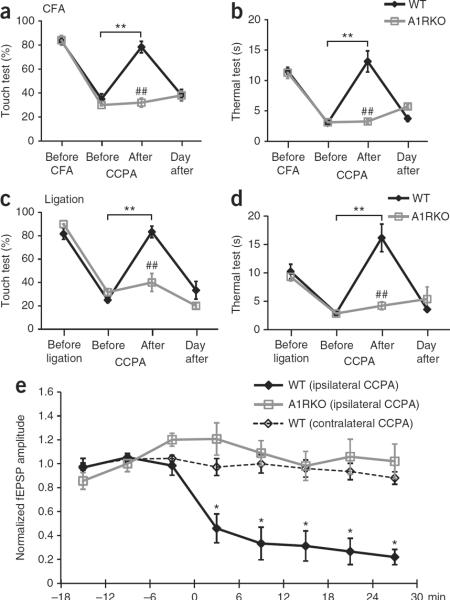
Having established that adenosine is released during acupuncture, we next asked whether adenosine is critical for the anti-nociceptive effects of acupuncture. We tested the effect of the selective A1 receptor agonist, 2-chloro-N(6)-cyclopentyladenosine (CCPA)19, in two mouse models of chronic pain. Inflammatory pain was evoked by injection of complete Freund's adjuvant (CFA) into the right paw20. Following injection of CFA, the mice developed mechanical allodynia to innocuous stimulation with Von Frey filaments of the ipsilateral paw peaking at day 4 to 5. The mice also developed thermal allodynia, as defined by a substantial decrease in withdrawal latency to heat. Administration of CCPA (0.1 mM, 20 μl) in the ipsilateral Zusanli point (ST36) evoked a sharp increase in the threshold to touch (Fig. 2a) and thermal pain (Fig. 2b). Touch sensitivity, defined as the percent of negative responses, improved from 35.0 ± 4.3 to 78.3 ± 4.8% (P < 0.01, Tukey-Kramer). Thermal sensitivity was almost abolished, with paw withdrawal time increasing from 3.0 ± 0.2 to 13.1 ± 1.7 s (P < 0.01) following administration of CCPA. Similarly, mechanical allodynia was sharply reduced by CCPA in mice with neuropathic pain (touch sensitivity improved from 25.0 ± 2.2 to 83.3 ± 4.9%, P < 0.01), concurrent with a reduction of the sensitivity to thermal pain (withdrawal of foot increased from 2.9 ± 0.3 to 16.2 ± 2.4 s, P < 0.01). To address whether A1 receptor signaling was sufficient for the anti-nociceptive effect of CCPA, we examined the effects of CCPA in mice lacking adenosine receptor A1 (ref. 21) and found that A1 receptor expression was necessary for CCPA-mediated pain suppression. Although CCPA effectively reduced mechanical and thermal hypersensitivity in wild-type mice, CCPA had no clinical benefit in mice lacking A1 receptors (Fig. 2a,b). Thus, the anti-nociceptive effects of CCPA require adenosine A1 receptor expression.
Figure 2.
Anti-nociceptive effects of adenosine A1 receptors. (a,b) Comparison of the effect of CCPA on mechanical allodynia (touch test, a) and thermal hyperalgesia (thermal test, b) in wild-type (WT, black) and A1 receptor knockout (A1RKO, gray) mice. CFA was administered in the right paw at day 0. The adenosine A1 receptor agonist CCPA (0.1 mM, 20 μl) was injected into the ipsilateral (right) Zusanli point (ST36) at day 4. All of the mice were evaluated ~10 min after the CCPA injection (**P < 0.01, Tukey-Kramer test compared with before CCPA; ##P < 0.01, comparison of wild type and A1RKO at each time point; n = 5−9). (c,d) Effect of CCPA on mechanical (c) and thermal hypersensitivity (d) in wild-type and A1RKO mice with neuropathic pain evoked by partial ligation of the right leg ischias nerve at day 0 and CCPA administered at day 6 (n = 6). The sensitivity of the contralateral (control) leg to mechanical and thermal stimulation from these experiments is shown in Supplementary Figure 1. (e) The amplitude of fEPSP in left anterior cingulate cortex evoked by painful stimulation in the right foot. The effect of CCPA (0.1 mM, 20 μl, at 0 min) injected in the ipsilateral (right) or the contralateral (left) Zusanli point on fEPSP is plotted as a function of time in wild-type and A1RKO mice. (*P < 0.01, Tukey-Kramer test compared with the −18- to −12-min time point, n = 4−12). Error bars indicate s.e.m.
We next modeled neuropathic pain by spared injury of the sciatic nerve22, in which pain peaked 5–7 d after nerve ligation. CCPA injected in the Zusanli point of the ipsilateral leg reduced neuropathic pain with an efficacy that was comparable to its suppression of inflammatory pain (Fig. 2c,d). In each model, the anti-nociceptive effect of CCPA was transient and did not alter sensitivity to painful stimulation in the contralateral leg (Supplementary Fig. 1). In addition, injection of CCPA into the contralateral leg did not alter the pain threshold of the ipsilateral leg, suggesting that the action of CCPA is mediated by activation of local A1 receptors (Supplementary Fig. 2). Substituting CCPA injection in the ipsilateral leg with an equal volume of saline (control vehicle) failed to change the threshold to either thermal or mechanically induced pain (Supplementary Fig. 2).
To understand how CCPA reduced sensitivity to painful stimulation and to specifically address whether CCPA acted directly on ascending nerve tracks, we recorded in vivo responses of the left anterior cingulate cortex (ACC) to painful stimulation of the right foot (Fig. 2e). The ACC is important for perception of pain23 and painful electrical nerve stimulation in humans is linked to its activation24. We found that high-intensity stimulation (10 mA, 20 ms) evoked consistent field excitatory postsynaptic potentials (fEPSPs) in the ACC with a latency of ~40 ms, reflecting the involvement of a polysynaptic pathway, which includes primary afferents and spinothalamic and thalamocortical tracts. Lower stimulation intensities evoked either no or variable responses, consistent with the idea that that ACC neurons respond primarily to painful stimuli24. After recording the responses to foot shock during baseline conditions for 20 min, we injected CCPA (0.1 mM, 20 μl) into the Zusanli point of the left leg, that is, contralateral to the foot receiving the painful stimuli. CCPA administered contralateral to the painful stimulation had no effect on fEPSPs, excluding the possibility that CCPA acted centrally (Fig. 2e). In contrast, CCPA injected in the Zusanli point in the right leg, ipsilateral to the painful stimulation, induced a marked decrease in the fEPSP amplitude. The decline of the fEPSP amplitude was observed as soon as 6 min after the CCPA injection, at which point the fEPSP amplitude fell from an average of ~0.65 mV before injection to ~0.22 mV within 20 min, representing a drop to 26.6 ± 11.0% of baseline values. Together, these data suggest that CCPA acts locally, probably on unmyelinated C fibers in the superficial peroneal nerve, which travels in close proximity to the Zusanli points. It has previously been documented that dorsal root ganglion cells express high levels of A1 receptors in their afferent terminals in the foot13,25 and in their presynaptic terminals in the substantia gelatinosa26. However, it seems unlikely that CCPA can diffuse over a distance of 1.8–2.0 mm and bind to A1 receptors in the foot or to the presynaptic terminals in the spinal cord located more than 3.0–3.2 mm from the Zusanli point in 6 min13,25,26. In sharp contrast with the potent depression of the amplitude of fEPSP in wild-type mice, mice with deletion of A1 receptors failed to respond to CCPA injection (Fig. 2e). Together, these studies suggest that CCPA reduced painful stimulation by activating adenosine A1 receptors on unmyelinated C fibers, and possibly A∂ fibers, in the superficial peroneal nerve.
Anti-nociceptive effect of acupuncture requires A1 receptors
We next asked whether adenosine released during acupuncture mediates the anti-nociceptive effects of acupuncture by evaluating the effects of acupuncture on inflammatory and neuropathic pain. A needle was gently inserted 1.5 mm deep in the ipsilateral Zusanli point and rotated once every 5 min for 30 min to mimic a typical acupuncture session. Animals with inflammatory pain clearly benefited from the acupuncture treatment; touch sensitivity rose from 22.2 ± 3.6 to 71.1 ± 3.5% (P < 0.01, Tukey-Kramer), whereas the threshold to thermal pain increased from 3.9 ± 0.4 to 10.6 ± 0.8 s (P < 0.01) (Fig. 3a,b). Similarly, acupuncture sharply reduced mechanical allodynia in mice suffering from neuropathic pain, with touch sensitivity improving from 26.7 ± 4.9 to 71.7 ± 4.8% (P < 0.01, Tukey-Kramer), and reduced sensitivity to thermal pain (withdrawal of foot increased from 3.1 ± 0.5 to 11.4 ± 0.9 s, P < 0.01, Tukey-Kramer; Fig. 3c,d). As with CCPA injection (Fig. 2) and consistent with earlier publications4, acupuncture-mediated pain suppression was transient in that hypersensitivity to both tactile and thermal stimulation returned to pre-acupuncture levels by the following day. Notably, acupuncture failed to reduce pain in A1 knockout mice. Hypersensitivity to either mechanical or thermal pain persisted in mice with deletion of adenosine A1 receptors in contrast with their littermate wild-type controls, which clearly benefitted from acupuncture (Fig. 3a–d). Acupuncture did not alter the pain sensitivity of the contralateral leg (Supplementary Fig. 1). Moreover, needle insertion without intermittent rotation stimulation failed to reduce pain sensitivity in both legs (Supplementary Fig. 3).
Figure 3.
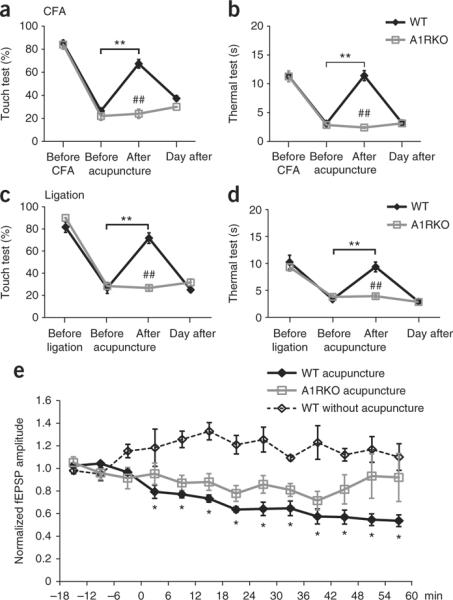
Acupuncture fails to suppress pain in mice lacking adenosine A1 receptors. (a,b) Acupuncture reduced sensitivity to both mechanical (a) and thermal (b) stimulation in wild-type mice suffering from inflammatory pain after injection of CFA in the right paw, but not in A1RKO littermates tested at day 4. All of the mice were evaluated ~10 min after acupuncture (**P < 0.01, Tukey-Kramer test compared with before acupuncture; ##P < 0.01, comparison of wild type and A1RKO at each time point, n = 5−9). (c,d) Acupuncture suppressed mechanical allodynia (c) and thermal hyperalgesia (d) in wild-type mice, but not in A1RKO mice, suffering from neuropathic pain. Neuropathic pain was induced by partial ligation of the right leg ischias nerve and the clinical effect of acupuncture tested at day 6 (n = 6). The sensitivity of the contralateral (control) leg to mechanical and thermal stimulation from these experiments is shown in Supplementary Figure 1. (e) The effect of acupuncture (0–30 min) on fEPSP amplitude in the left anterior cingulate cortex evoked by painful stimulation in the right leg. fEPSP amplitude is plotted as a function of time in wild-type and A1RKO mice (*P < 0.01, Tukey-Kramer test compared with the −18- to −12-min time points, n = 3−8). Error bars indicate s.e.m.
A remaining question is whether adenosine released during acu-puncture, similar to CCPA, reduces input to the ACC in response to painful stimulation. To this end, we used a similar strategy as above (Fig. 2) to assess the effect of acupuncture on the amplitude of fEPSPs recorded in the left ACC that were evoked by painful stimulation in the right leg (Fig. 3e). Similar to CCPA injection, acupuncture in the left Zusanli point (contralateral to the stimulation) had no effect on the fEPSP in response to painful stimulation (data not shown). However, acupuncture in the right Zusanli point (ipsilateral to the stimulation) suppressed fEPSP and the inhibition continued to increase in potency during the observation period. fEPSP amplitude was maximally reduced to 53.7 ± 7.2% (P < 0.01) of baseline at 60 min (Fig. 3e). On the other hand, acupuncture did not alter fEPSP in mice with deletion of A1 receptors. Combined, these observations provide direct evidence for a role of adenosine in acupuncture-mediated anti-nociceptive effects in models of inflammatory and neuropathic pain. The relatively slow time course of fEPSP depression, compared with the sharp decrease in fEPSP amplitude following CCPA injection, suggests that adenosine slowly accumulated in the extracellular space during acupuncture (Figs. 2e and 3e). In addition, pathways other than A1 receptors may contribute to acupuncture-mediated pain suppression. These mechanisms could include direct action of ATP on P2X receptors, as has been recently proposed, or central release of opioid peptides4,27. The lack of pro-nociceptive, hyperalgesic effects of A2A and A3 receptor activation (except for the immediate pain felt during the needle insertion) can be ascribed to the low expression of these receptors in muscle28. Mice with deletion of A2A receptors exhibited no benefit of either CCPA injection or acu-puncture (Supplementary Fig. 4) compared with wild-type controls (Figs. 2 and 3)29.
Manipulation of AMP metabolism prolongs acupuncture effect
Similar to other types of tissue injury, accumulation of nucleotides in the interstitial space during acupuncture is probably a consequence of unspecific membrane damage or opening of stress-activated channels9. The relatively high extracellular concentration of ATP metabolites compared with ATP (Fig. 1c) likely reflects the rapid enzymatic degradation of ATP by ectonucleotidases, as ATP is present in the cytosol of skeletal muscles, fibroblasts and fat cells in a concentration of 4–8 mM, or about 100-fold higher than AMP and adenosine30. Thus, it may be possible to potentiate acupuncture-induced increases in the adenosine by manipulating the enzymatic pathways involved in catabolism of extracellular ATP. We asked which catabolic step is rate-limiting for the extracellular degradation of ATP to adenosine. Using sections of tissue (muscle and subcutis) harvested at the Zusanli point, we found that phosphate was generated at a rate of 0.428 ± 0.046 μM mg−1 min−1 when 1 mM ATP was added as substrate, whereas addition of AMP (1 mM) produced phosphate at a rate of only 0.043 ± 0.005 μM mg−1 min−1 (n = 3, P < 0.01, Student's t test). The slow kinetics of the latter reaction indicates that AMP dephosphorylation is the rate-limiting step in adenosine production (Supplementary Fig. 5). A detailed analysis suggested that multiple enzymes, including prostatic acid phosphatase31,32, as well as not yet identified phosphatases, contributed to extracellular formation of adenosine in muscle/subcutis (Supplementary Fig. 5). However, AMP is not necessarily degraded to adenosine, as AMP in skeletal muscles also can be deaminated to inosine monophosphate (IMP) by AMP deaminase33 (Fig. 4a). Consistent with a prior report33, we found that IMP was generated in much larger quantities than adenosine when AMP was added as the substrate (Fig. 4b). AMP deaminase functions as an enzymatic shuttle for degradation of AMP that bypasses adenosine production (Fig. 4a). On the basis of this observation, we asked whether it is possible to increase and/or prolong acupuncture-induced increases in adenosine by inhibiting AMP deaminase activity. We used the nucleoside analog deoxycoformycin, which inhibits AMP deaminase and adenosine deaminase34 and thereby suppresses the two major pathways involved in elimination of extracellular adenosine (Fig. 4a). Consistent with an inhibitory effect of deoxycoformycin on both deaminases, we found that deoxycoformycin significantly increased the accumulation of adenosine (2.9-fold increase, P = 0.025) and suppressed IMP (0.08-fold decrease, P = 0.023) and inosine (0.24-fold decrease, P = 0.005) production in isolated samples of muscle/subcutis harvested at the Zusanli point (Fig. 4b).
Figure 4.
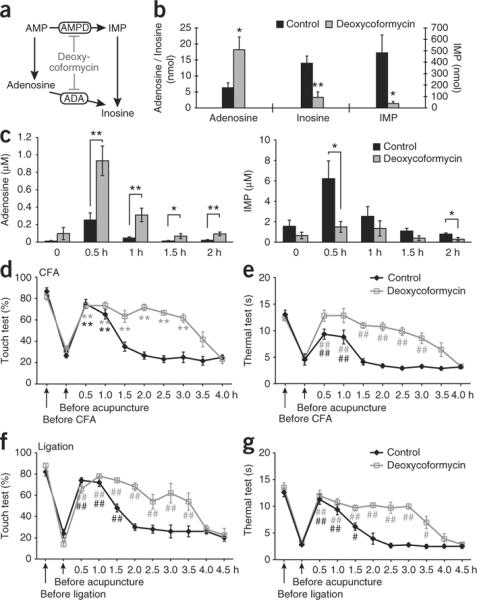
Pharmacological inhibition of deaminase activity enhances increases in adenosine and prolongs anti-nociception actions of acupuncture. (a) Schematic diagram outlining the two major pathways involved in extracellular enzymatic degradation of AMP. The nucleoside analog deoxycoformycin inhibits both AMP deaminase (AMPD) and adenosine deaminase (ADA). (b) Histogram comparing the production of adenosine, IMP and inosine when tissue sections harvested close to the Zusanli point were incubated in 1 mM AMP and an inhibitor of adenosine uptake, nitrobenzylthioinosine (100 μM), for 45 min. Deoxycoformycin (500 μM) increased accumulation of adenosine while inhibiting the production of IMP and inosine (*P < 0.05, **P < 0.01, t test comparison between control and deoxycoformycin, n = 5). (c) Analysis of microdialysis samples collected close to the Zusanli point in mice treated with deoxycoformycin (50 mg per kg, intraperitoneal) or vehicle (saline). Deoxycoformycin increased accumulation of adenosine, while inhibiting the production of IMP in vivo during and after acupuncture (n = 6−8). (d,e) Deoxycoformycin (50 mg per kg, intraperitoneal) prolonged the anti-nociceptive effect of acupuncture in wild-type mice with inflammatory pain in response to mechanical (d) and thermal (e) stimulation (##P < 0.01, Tukey-Kramer test compared with before acupuncture, n = 6−10). (f,g) Deoxycoformycin prolonged the anti-nociceptive effect of acupuncture in wild-type mice with neuropathic pain induced by partial ligation of the ischias nerve to mechanical (f) and to thermal (g) stimulation (#P < 0.05, ##P < 0.01, Tukey-Kramer test compared with before acupuncture, n = 5). The sensitivity of the contralateral (control) leg to mechanical and thermal stimulation from these experiments is shown in Supplementary Figure 1. Error bars indicate s.e.m.
To assess the clinical potential of systemic administration of deoxycoformycin as an adjuvant to acupuncture, we treated mice with deoxycoformycin (50 mg per kg of body weight, intraperitoneal) and collected microdialysis samples 0.4–0.6 mm from the Zusanli point. Mice receiving deoxycoformycin exhibited a sharp increase in the extracellular accumulation of adenosine during acupuncture (3.68-fold increase, P = 0.0081; Fig. 4c). Moreover, deoxycoformycin prolonged the accumulation of adenosine, which remained significantly elevated (0.5, 1 and 2 h, P < 0.01; 1.5 h, P < 0.05, t test) for the duration of the experiment (Fig. 4c). The increases in acupuncture-induced adenosine accumulation was, similar to the ex vivo observations, mirrored by a decrease of IMP consistent with an inhibitory effect of deoxycoformycin on AMP deaminase (Fig. 4c).
The fact that deoxycoformycin potentiated and prolonged adenosine increases induced by acupuncture raises the question of whether deoxycoformycin can be used as an adjuvant to acupuncture, which potentiate the anti-nociception. To address this point, we compared the anti-nociceptive effect of acupuncture in mice to that received deoxycoformycin (50 mg per kg, intraperitoneal) versus vehicle (phosphate-buffered saline). The 30-min acupuncture session (needle rotated twice every 5 min for a total of 30 min) reduced pain for a duration of ~1.0–1.5 h in mice that received vehicle (Fig. 4d–g). Notably, mice pretreated with deoxycoformycin exhibited a significant prolongation (P < 0.05, Tukey-Kramer test compared with before acupuncture) of anti-nociception. Mechanical allodynia and thermal pain sensitivity were suppressed for ~3.0–3.5 h in mice suffering from either inflammatory or neurogenic pain, when deoxycoformycin was added as an adjuvant to acupuncture (Fig. 4d–g). Deoxycoformycin did not alter the pain sensitivity of the contralateral leg (Supplementary Fig. 1). In addition, deoxycoformycin had no effect on either the tactile or thermal sensitivity when it was not combined with acupuncture in the two models of chronic pain (Supplementary Fig. 6) consistent with the fact that deoxycoformycin failed to significantly increase (P = 0.1728, t test comparison between control and deoxycoformycin) the resting, unstimulated concentration of adenosine. These data suggest that suppression of deaminase activity can be used as an adjuvant to acupuncture, which effectively increases its clinical benefits. Deoxycoformycin (Pentostatin) is an antimicrobial nucleoside analog that inhibits DNA synthesis and is approved by the Food and Drug Administration for treatment of leukemia35.
DISCUSSION
Although acupuncture has been practiced for over 4,000 years, it has been difficult to establish its biological basis. Our findings indicate that adenosine is central to the mechanistic actions of acupuncture. We found that insertion and manual rotation of acupuncture needles triggered a general increase in the extracellular concentration of purines, including the transmitter adenosine (Fig. 1), which is consistent with the observation that tissue damage is associated with an increase in extracellular nucleotides and adenosine36. Because the anti-nociceptive effects of peripheral, spinal and supraspinal adenosine A1 receptors are well established37,38, we asked whether peripheral injection of an A1 receptor agonist suppressed hyper-algesia25,37 (Fig. 2). We found that the A1 receptor agonist CCPA sharply reduced inflammatory and neurogenic pain and that suppression of pain mediated by acupuncture required adenosine A1 receptor expression (Fig. 3). These findings suggest that A1 receptor activation is both necessary and sufficient for the clinical benefits of acupunctures. To the best of our knowledge, adenosine A1 receptors have not previously been implicated in the anti-nociceptive actions of acupuncture.
One may speculate that other non-allopathic treatments of chronic pain, such as chiropractic manipulations and massage, modalities that involve the mechanical manipulation of joints and muscles, might also be associated with an efflux of cytosolic ATP that is sufficient to elevate extracellular adenosine. As in acupuncture, adenosine may accumulate during these treatments and dampen pain in part by the activation of A1 receptors on sensory afferents of ascending nerve tracks. Notably, needle penetration has been reported to not confer an analgesic advantage over nonpenetrating (placebo) needle application39, as opposed to our observations (Supplementary Figs. 2 and 3) and those of others40,41. However, it is possible that ATP release from keratinocytes in response to mechanical stimulation of the skin results in an accumulation of adenosine that transiently reduces pain, as A1 receptors are probably expressed by nociceptive axon terminal in epidermis37. In fact, vibratory stimulation applied to the skin depressed the activity of nociceptive neurons in the lower lumbar segments of cats by release of adenosine42. However, this effect differs from the anti-nociceptive effect of acupuncture, which does not depend on the afferent innervation of the skin4. Acupuncture is typically applied to deep tissue, including muscle and connective tissue, and acupoints may better overlap with their proximity to ascending nerve tracks than to the density of cutaneous afferents.
Most patients have reported that acupuncture in itself is not a painful procedure, except for a pinching sensation in tissue below the acupuncture needle. Because ATP is released during acupuncture (Fig. 1), the pinching sensation may be mediated by nociceptive P2X3 receptors, which are expressed by small-diameter, primary afferent neurons, some of which are sensitive to capsaicin10. The most likely explanation for the lack of direct pain during acupuncture is that extracellular ATP does not reach high enough concentrations to activate P2X3 and other nociceptive P2X receptors because of its rapid degradation (Fig. 1). However, activation of P2X receptors may nonetheless contribute to the anti-analgesic effects of acupuncture, as was recently suggested27, perhaps by subthreshold-activating P2X receptors or by more complex mechanisms involving dimerization of P2X and A1 receptors43. In addition to the use of acupuncture for treatment of chronic pain, acupuncture is also frequently employed in diseases with a local inflammatory component, such as arthritis and tendinitis44. Adenosine has anti-inflammatory properties and we found that acupuncture increased extracellular adenosine36 (Fig. 1).
Quantification of extracellular purines in microdialysis samples collected nearby the acupuncture point revealed that the extracellular adenosine concentration rose following the release of ATP, which was dephosphorylated to ADP, AMP and adenosine by potent ectonucleotidases, and that AMP dephosphorylation represents the rate-limiting step in this reaction (Supplementary Fig. 5). As with most other transmitters, adenosine has a short lifespan in the extracellular space as a result of facilitated uptake by nucleoside transporters and concurrent degradation to inosine33. After reuptake, adenosine is quickly converted to AMP by cytosolic adenosine kinase (Km ~20 nM), thereby facilitating the rapid clearance of adenosine in the extracellular space36 and shortening the anti-nociceptive effects of acupuncture. Moreover, our analysis confirmed the prior observation that AMP deaminase activity is high in muscle/subcutis33 and that only a fraction of AMP is dephosphorylated to adenosine. AMP deaminases constitute the primary enzymatic pathway for elimination of extracellular AMP and this pathway bypasses adenosine. Thus, acupuncture combined with pharmacological suppression of AMP deaminase activity should theoretically increase the availability of adenosine and thereby enhance the clinical benefits of acupuncture. As a proof of principle, we found that mice treated with a Food and Drug Administration approved deaminase inhibitor, deoxycoformycin, exhibited more potent increases in adenosine and benefitted from a longer-lasting suppression of chronic pain following acupuncture. In summary, we found that the anti-nociceptive action of acupuncture is mediated by activation of A1 receptors located on ascending nerves. Thus, medications that interfere with A1 receptors or adenosine metabolism may improve the clinical benefit of acupuncture.
ONLINE METHODS
Surgery, experimental models, behavioral assessment, CCPA administration, acupuncture and immunohistochemistry
The Institutional Animal Care and Use Committee at University of Rochester approved all of the procedures used in this study. As per the direction of the International Society for the Study of Pain guidelines, we attempted to use the minimum number of mice necessary to achieve statistical significance. C57BL/6J male mice (8–14 weeks old, Jackson Labs) were used in all experiments. A1 receptor knockout mice21, A2A receptor knockout mice29, and CD73 knockout mice45 were on C57BL/6 genetic background and wild-type littermate used as controls.
Peripheral inflammation was induced by injection of CFA (mixed with an equal amount of oil, total volume 0.1 ml) in the plantar surface of the right hind paw of mice20. An equal amount of saline (0.1 ml) was injected in the left hind paw as a control. Neuropathic pain was induced by ligation of the right leg sciatic nerve with 4.0 polypropylene suture in mice anesthetized with ketamine (60 mg per kg, intraperitoneal) and xylazine (10 mg per kg, intraperitoneal)46.
Mechanical allodynia was evaluated using repeated stimulations with a Von Frey filament exerting 0.02 g of force onto the plantar surface. The percentage of negative responses of a total of ten trials was calculated for each foot. Thermal hyperalgesia was assessed using an Analgesymeter (Ugo Basile). A mobile radiant heat source was focused on the hind paw and the paw withdrawal latencies were defined as the time taken by the mouse to remove its hind paw from the heat source (maximum of 20 s to avoid tissue damage). The paw withdrawal was repeated three times for each foot and the average calculated. To avoid conditioning to stimulation, we interposed a 5-min rest period between each trial in both thermal and mechanical tests. Behavioral parameters were evaluated before intraplantar injection of CFA or nerve ligation (that is, day 0), on day 3–4 in mice receiving the CFA injection, and at day 5–7 in mice with nerve ligation, unless otherwise noted. Prior to injection of CCPA (0.1 mM, 20 μl), saline or acupuncture, the mice were restrained under light isoflurane anesthesia (~1%). All the mice were evaluated ~10 min after the CCPA injection or acupuncture. The total duration of anesthesia was ~2 min and mice with inflammatory or neurogenic pain and their controls were treated similarly. For acupuncture, a small acupuncture needle, 0.16 × 13 mm (08–02, Lhass Medical), was gently inserted in a depth of 1.5 mm in the Zusanli point (ST36) located 3–4 mm below and lateral 1–2 mm for the midline of the knee4. The needle was slowly rotated every 5 min for a total of 30 min during an acupuncture session. We chose to study the effect of acupuncture on chronic pain applied in the acupoint Zusanli because it is one of the most effective points in traditional Chinese medicine and its antinociceptive effects in rodent models of chronic pain are well-established4. For HPLC analysis of purine releases in sections of muscle/subcutis, a microdialysis probe (MD-2211, Bioanalytical Systems) was implanted 1.5 h before collection of microdialysis samples. The microdialysis probe was implanted 0.4–0.6 mm from the Zusanli point. The microdialysis probe was perfused with Ringer's solution at a rate of 1 μl per min. The microdialysates were collected on ice over a 30-min period (30 μl) and were immediately frozen at −80 °C until HPLC analysis. Vehicle (saline, intraperitoneal) or deoxycoformycin (50 mg per kg, intraperitoneal) was administered 30 min before acupuncture. Immunohistochemistry was performed as previously described18.
In vivo electrophysiology
Mice were anesthetized with 2–3% isoflurane (vol/vol), intubated and artificially ventilated with a small animal ventilator (SAAR-830, CWE). Body temperature was monitored by a rectal probe and maintained at 37 °C by a heating blanket (BS4, Harvard Apparatus). A craniotomy (1–1.5 mm in diameter), centered 0.1 mm anterior to the bregma and 1.5 mm lateral from midline, was made over the left ACC. A custom-made metal plate was glued to the skull with dental acrylic cement. The mice were maintained at 2 vol% isoflurane for the remainder of the experiment. Local field potential recordings were obtained from layer 4 of ACC, 0.8 mm below the pial surface by a patch pipette (TW100F-4, WPI; outer diameter, 1.0 mm; inner diameter, 0.75 mm; tip diameter, 1–2 μm). Local field potential signals were amplified, bandpass filtered at (1–100 Hz) and digitized at 10 kHz as described previously18. Dura matter was kept intact. A custom-made bipolar electrode was inserted subcutaneously into the right hindpaw. High-intensity stimulation (10 mA, 20 ms) was evoked every 120 s. Lower stimulation intensities evoked either no or variable responses, consistent with the idea that that ACC neurons respond primarily to painful stimuli23. fEPSP amplitude was measured using pCLAMP 9.2 program (Axon Instruments). We chose to record fEPSPs evoked by painful stimulation in the ACC rather than in the substantia gelatinosa of the spinal cord to detect systemic effect of CCPA injected in the contralateral (left) leg.
HPLC analysis of purines
The analysis of enzymatic degradation of purines was based on sections (~4 mm) of skeletal muscles with overlying subcutis harvested from tissue below the Zusanli point. The sections were incubated with O2-bubbled buffer (125 mM NaCl, 5 mM KCl, 1.25 mM NaH2PO4, 2 mM MgSO4, 2 mM CaCl2, 10 mM glucose and 25 mM NaHCO3, pH 7.3, gassed with 95% O2 and 5% CO2) for 30 min. Each section per well was placed into a 6-well plate in 3 ml Hanks' balanced salt solution with 15 mM HEPES (pH 7.3) containing 1 mM AMP with or without 500 μM deoxycoformycin (Tocris Bioscience). The adenosine transport inhibitor nitrobenzylthioinosine (Sigma) was added to all samples to reduce uptake of adenosine. The samples were collected from each well at 0 and 45 min and stored at −80 °C for HPLC analysis. The analyses were carried out using CoulArray 5600A system (ESA) and an ESA model 526 ultraviolet detector as described previously14,15. Chromatographic separation was achieved by using a reverse-phase column (Lichrospher 100 RP-18, 5 μm, 250 mm × 3 mm, Merck). For measurements of microdialysates, we used a mobile phase consisting of 215 mM KH2PO4, 2.3 mM tetrabutylammonium bisulfate, 3.2% acetonitrile (vol/vol, HPLC-grade water), pH 6.2. For measurements of samples prepared from muscle/subcutis sections, we used a mobile phase consisting 215 mM KH2PO4, 1.2 mM tetrabutylammonium bisulfate, 1% acetonitrile, pH 6.0. The flow rate was maintained at 0.25 ml min−1. Daily calibration curves were prepared by a four-point standard (3, 1, 0.3 or 0.1 μM) of ATP, ADP, AMP, adenosine, inosine and IMP in 0.4 M perchloric acid, respectively. Eluted purines were detected at 260 nm and the chromatographic peaks were integrated using CoulArray software. Notably, deoxycoformycin-induced changes in inosine concentration could not be evaluated in microdialysis samples, as the peak of deoxycoformycin overlapped with inosine. Pharmacological analysis of enzymes involved in extracellular dephosphorylation of AMP or ATP was measured using the Malachite Green Phosphate Detection Kit (Sigma) in samples collected from sections incubated in a phosphate-free Ringer solution. All measurements were normalized to wet weight in milligrams.
Statistical analysis
Statistical analyses were carried out with ANOVA with Tukey-Kramer post hoc test. Where only two groups were compared, Student's t test was used.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the US National Institutes of Health to M.N. and K.T.
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
AUTHOR CONTRIBUTIONS All authors contributed to experimental design and execution and manuscript preparation.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
References
- 1.NIH Consensus Conference Acupuncture. J. Am. Med. Assoc. 1998;280:1518–1524. [PubMed] [Google Scholar]
- 2.Culliton BJ. Acupuncture: fertile ground for faddists and serious NIH research. Science. 1972;177:592–594. doi: 10.1126/science.177.4049.592. [DOI] [PubMed] [Google Scholar]
- 3.Bonafede M, Dick A, Noyes K, Klein JD, Brown T. The effect of acupuncture utilization on healthcare utilization. Med. Care. 2008;46:41–48. doi: 10.1097/MLR.0b013e3181589b7d. [DOI] [PubMed] [Google Scholar]
- 4.Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog. Neurobiol. 2008;85:355–375. doi: 10.1016/j.pneurobio.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Han JS. Acupuncture and endorphins. Neurosci. Lett. 2004;361:258–261. doi: 10.1016/j.neulet.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Han JS, Wan Y. Characteristics of electroacupuncture-induced analgesia in mice: variation with strain, frequency, intensity and opioid involvement. Brain Res. 2002;945:20–25. doi: 10.1016/s0006-8993(02)02503-9. [DOI] [PubMed] [Google Scholar]
- 7.Lao L, et al. A parametric study of electroacupuncture on persistent hyperalgesia and Fos protein expression in rats. Brain Res. 2004;1020:18–29. doi: 10.1016/j.brainres.2004.01.092. [DOI] [PubMed] [Google Scholar]
- 8.Li WM, et al. Analgesic effect of electroacupuncture on complete Freund's adjuvant-induced inflammatory pain in mice: a model of antipain treatment by acupuncture in mice. Jpn. J. Physiol. 2005;55:339–344. doi: 10.2170/jjphysiol.RP001505. [DOI] [PubMed] [Google Scholar]
- 9.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signaling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 11.Sjölund KF, Segerdahl M, Sollevi A. Adenosine reduces secondary hyperalgesia in two human models of cutaneous inflammatory pain. Anesth. Analg. 1999;88:605–610. doi: 10.1097/00000539-199903000-00027. [DOI] [PubMed] [Google Scholar]
- 12.Maione S, et al. The antinociceptive effect of 2-chloro-2′-C-methyl-N6-cyclopentyladenosine (2′-Me-CCPA), a highly selective adenosine A1 receptor agonist, in the rat. Pain. 2007;131:281–292. doi: 10.1016/j.pain.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Sawynok J, Reid A, Poon A. Peripheral antinociceptive effect of an adenosine kinase inhibitor, with augmentation by an adenosine deaminase inhibitor, in the rat formalin test. Pain. 1998;74:75–81. doi: 10.1016/S0304-3959(97)00153-X. [DOI] [PubMed] [Google Scholar]
- 14.Cui M, Tang X, Christian WV, Yoon Y, Tieu K. Perturbations in mitochondrial dynamics induced by human mutant PINK1 can be rescued by the Mitochondrial Division Inhibitor mdivi-1. J. Biol. Chem. 2010;285:11740–11752. doi: 10.1074/jbc.M109.066662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volonté MG, Yuln G, Quiroga P, Consolini AE. Development of an HPLC method for determination of metabolic compounds in myocardial tissue. J. Pharm. Biomed. Anal. 2004;35:647–653. doi: 10.1016/j.jpba.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Li J, King NC, Sinoway LI. Interstitial ATP and norepinephrine concentrations in active muscle. Circulation. 2005;111:2748–2751. doi: 10.1161/CIRCULATIONAHA.104.510669. [DOI] [PubMed] [Google Scholar]
- 17.Li J, King NC, Sinoway LI. ATP concentrations and muscle tension increase linearly with muscle contraction. J. Appl. Physiol. 2003;95:577–583. doi: 10.1152/japplphysiol.00185.2003. [DOI] [PubMed] [Google Scholar]
- 18.Bekar L, et al. Adenosine is crucial for deep brain stimulation-mediated attenuation of tremor. Nat. Med. 2008;14:75–80. doi: 10.1038/nm1693. [DOI] [PubMed] [Google Scholar]
- 19.Lohse MJ, et al. 2-Chloro-N6-cyclopentyladenosine: a highly selective agonist at A1 adenosine receptors. Naunyn Schmiedebergs Arch. Pharmacol. 1988;337:687–689. doi: 10.1007/BF00175797. [DOI] [PubMed] [Google Scholar]
- 20.Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant–induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur. J. Neurosci. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- 21.Sun D, et al. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc. Natl. Acad. Sci. USA. 2001;98:9983–9988. doi: 10.1073/pnas.171317998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vadakkan KI, Jia YH, Zhuo M. A behavioral model of neuropathic pain induced by ligation of the common peroneal nerve in mice. J. Pain. 2005;6:747–756. doi: 10.1016/j.jpain.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Wei F, Zhuo M. Potentiation of sensory responses in the anterior cingulate cortex following digit amputation in the anaesthetized rat. J. Physiol. (Lond.) 2001;532:823–833. doi: 10.1111/j.1469-7793.2001.0823e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ. Functional MRI of pain- and attention-related activations in the human cingulate cortex. J. Neurophysiol. 1997;77:3370–3380. doi: 10.1152/jn.1997.77.6.3370. [DOI] [PubMed] [Google Scholar]
- 25.Karlsten R, Gordh T, Post C. Local antinociceptive and hyperalgesic effects in the formalin test after peripheral administration of adenosine analogues in mice. Pharmacol. Toxicol. 1992;70:434–438. doi: 10.1111/j.1600-0773.1992.tb00503.x. [DOI] [PubMed] [Google Scholar]
- 26.Reeve AJ, Dickenson AH. Electrophysiological study on spinal antinociceptive interactions between adenosine and morphine in the dorsal horn of the rat. Neurosci. Lett. 1995;194:81–84. doi: 10.1016/0304-3940(95)11732-c. [DOI] [PubMed] [Google Scholar]
- 27.Burnstock G. Acupuncture: a novel hypothesis for the involvement of purinergic signaling. Med. Hypotheses. 2009;73:470–472. doi: 10.1016/j.mehy.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 28.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 29.Chen JF, et al. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J. Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poortmans J. Principles of Exercise Biochemistry. Karger; Brussels: 2003. [Google Scholar]
- 31.Quintero IB, et al. Prostatic acid phosphatase is not a prostate-specific target. Cancer Res. 2007;67:6549–6554. doi: 10.1158/0008-5472.CAN-07-1651. [DOI] [PubMed] [Google Scholar]
- 32.Zylka MJ, et al. Prostatic acid phosphatase is an ectonucleotidase and suppresses pain by generating adenosine. Neuron. 2008;60:111–122. doi: 10.1016/j.neuron.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cunha RA, Sebastiao AM. Extracellular metabolism of adenine nucleotides and adenosine in the innervated skeletal muscle of the frog. Eur. J. Pharmacol. 1991;197:83–92. doi: 10.1016/0014-2999(91)90368-z. [DOI] [PubMed] [Google Scholar]
- 34.Golembiowska K, White TD, Sawynok J. Modulation of adenosine release from rat spinal cord by adenosine deaminase and adenosine kinase inhibitors. Brain Res. 1995;699:315–320. doi: 10.1016/0006-8993(95)00926-h. [DOI] [PubMed] [Google Scholar]
- 35.Lamanna N, Kay NE. Pentostatin treatment combinations in chronic lymphocytic leukemia. Clin. Adv. Hematol. Oncol. 2009;7:386–392. [PubMed] [Google Scholar]
- 36.Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 37.Sawynok J. Adenosine receptor activation and nociception. Eur. J. Pharmacol. 1998;347:1–11. doi: 10.1016/s0014-2999(97)01605-1. [DOI] [PubMed] [Google Scholar]
- 38.Eisenach JC, et al. Intrathecal but not intravenous opioids release adenosine from the spinal cord. J. Pain. 2004;5:64–68. doi: 10.1016/j.jpain.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Takakura N, Yajima H. Analgesic effect of acupuncture needle penetration: a double-blind crossover study. Open Med. 2009;3:e54–61. [PMC free article] [PubMed] [Google Scholar]
- 40.Weidenhammer W, Linde K, Streng A, Hoppe A, Melchart D. Acupuncture for chronic low back pain in routine care: a multicenter observational study. Clin. J. Pain. 2007;23:128–135. doi: 10.1097/01.ajp.0000210952.09127.df. [DOI] [PubMed] [Google Scholar]
- 41.Kelly RB. Acupuncture for pain. Am. Fam. Physician. 2009;80:481–484. [PubMed] [Google Scholar]
- 42.Salter MW, Henry JL. Evidence that adenosine mediates the depression of spinal dorsal horn neurons induced by peripheral vibration in the cat. Neuroscience. 1987;22:631–650. doi: 10.1016/0306-4522(87)90359-9. [DOI] [PubMed] [Google Scholar]
- 43.Sichardt K, Nieber K. Adenosine A(1) receptor: functional receptor-receptor interactions in the brain. Purinergic Signal. 2007;3:285–298. doi: 10.1007/s11302-007-9065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zijlstra FJ, van den Berg-de Lange I, Huygen FJ, Klein J. Anti-inflammatory actions of acupuncture. Mediators Inflamm. 2003;12:59–69. doi: 10.1080/0962935031000114943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castrop H, et al. Impairment of tubuloglomerular feedback regulation of GFR in ecto-5′-nucleotidase/CD73-deficient mice. J. Clin. Invest. 2004;114:634–642. doi: 10.1172/JCI21851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martucci C, et al. The purinergic antagonist PPADS reduces pain related behaviors and interleukin-1 beta, interleukin-6, iNOS and nNOS overproduction in central and peripheral nervous system after peripheral neuropathy in mice. Pain. 2008;137:81–95. doi: 10.1016/j.pain.2007.08.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.