Abstract
Rationale
Electrical conduction through gap junction channels between endothelial cells of resistance vessels is integral to blood flow control. Small and intermediate-conductance Ca2+-activated K+ channels (SKCa/IKCa) initiate electrical signals in endothelial cells but it is unknown whether SKCa/IKCa activation alters signal transmission along the endothelium.
Objective
We tested the hypothesis that SKCa/IKCa activity regulates electrical conduction along the endothelium of resistance vessels.
Methods and Results
Freshly isolated endothelial cell tubes (60 μm wide; 1–3mm long; cell length, ~35 μm) from mouse skeletal muscle feed (superior epigastric) arteries were studied using dual intracellular microelectrodes. Current was injected (±0.1–3 nA) at Site 1 while recording membrane potential (Vm) at Site 2 (separation distance = 50–2000 μm). SKCa/IKCa activation (NS309, 1 μmol/L) reduced the change in Vm along endothelial cell tubes by ≥50% and shortened the electrical length constant (λ) from 1380 to 850 μm (P<0.05) while intercellular dye transfer (propidium iodide) was maintained. Activating SKCa/IKCa with acetylcholine or SKA-31 also reduced electrical conduction. These effects of SKCa/IKCa activation persisted when hyperpolarization (>30 mV) was prevented with 60 mM [K+]o. Conversely, blocking SKCa/IKCa (apamin + charybdotoxin) depolarized cells by ~10 mV and enhanced electrical conduction (i.e., changes in Vm) by ~30% (P<0.05).
Conclusions
These findings illustrate a novel role for SKCa/IKCa activity in tuning electrical conduction along the endothelium of resistance vessels by governing signal dissipation through changes in membrane resistance. Voltage-insensitive ion channels can thereby tune intercellular electrical signaling independent from gap junction channels.
Keywords: conducted vasodilation, hyperpolarization, gap junctions, potassium channels
Introduction
Electrical conduction through gap junction channels (GJCs) coordinates vasomotor responses in vascular resistance networks.1–3 Through axial orientation and robust expression of GJCs, endothelial cells (ECs) serve as the primary pathway for cell-to-cell conduction along the vessel wall4–6 with electrical signals transmitted to smooth muscle cells (SMCs) via myoendothelial GJCs.3, 7–9 Once initiated, hyperpolarization (and vasodilation) can travel along the resistance vasculature for millimeters.10–13 As shown in exercising skeletal muscle, vasodilation originating downstream within the microcirculation can ascend the resistance network via conduction along the endothelium to encompass feed arteries upstream and thereby increase total blood flowing into the active muscle.14
In accord with the cable properties of electrical conduction, the distance that signals travel along the endothelium is determined by the length constant (λ = rm/ra)1/2, where rm = membrane resistance and ra = axial resistance to current flow.15, 16 With negligible resistance of cytoplasm, intercellular ra is determined primarily by GJCs.8, 16 Previous studies have focused on alterations of conducted vasodilation through manipulating the functional integrity of GJCs5, 14, 17 or the expression profile of their connexin subunits.18–21 Alternatively, changes in rm may also provide a mechanism for governing electrical conduction independent of manipulating GJCs. In turn, changes in rm should reflect the activation of ion channels in the plasma membrane,15, 16 which otherwise insulates the cell interior from the extracellular milieu. Remarkably, the effect of governing rm through ion channel activation has not been studied in light of electrical conduction along the endothelium of resistance vessels.
The small (KCa 2.3; KCNN3)-and intermediate (KCa 3.1; KCNN4)-conductance Ca2+-activated K+ channels (SKCa/IKCa) are abundantly expressed in EC membranes.22–24 Upon activation of SKCa/IKCa the efflux of K+ promotes hyperpolarization.22, 25 While this response may promote vasodilation and tissue perfusion,24 a reduction in rm along the endothelium could dissipate electrical signals and thereby compromise electrical conduction and impair ascending vasodilation. Given the prominent role of SKCa/IKCa in the initiation of EC hyperpolarization,9, 24 we questioned whether activation of these ion channels impacts electrical conduction along the endothelium.
Understanding how the activation of SKCa /IKCa may govern electrical conduction along the endothelium of intact vessels is limited by myoendothelial coupling to SMCs, perivascular nerve activity, and circulating vasoactive agents along with the shear stress exerted by blood flow. Further, the initiation of an electrical signal to trigger conduction has typically consisted of an agonist (e.g., ACh4, 10, 26) or electrical field stimulation27, 28 via micropipettes. However, such “local” stimuli can activate multiple cells simultaneously, with uncertainty in the precise origin and intensity of the actual stimulus. To avoid such confounding influences, we have freshly isolated EC tubes from feed arteries of mouse abdominal skeletal muscle.29, 30 Intact EC tubes from these resistance vessels can exceed 3 mm in length, enabling the efficacy of electrical conduction along the endothelium to be evaluated. Using sharp intracellular microelectrodes, current microinjection into a single EC alters membrane potential (Vm) independent of agonists or ion channel activation. By simultaneously recording Vm at defined distances from the site of current injection, we tested the hypothesis that SKCa/IKCa activity can `tune' electrical conduction along the endothelium.
Findings reported here are the first to show that activating SKCa/IKCa with either pharmacological agents (NS309, SKA-31) or a physiological agonist (ACh) impairs electrical conduction along the endothelium of a resistance artery. Concomitantly, intercellular coupling through GJCs remains intact as confirmed by robust dye transfer between ECs. We further demonstrate that blocking constitutively open SKCa/IKCa enhances electrical conduction. Thus altering rm via opening and closing plasma membrane ion channels effectively tunes the conduction of electrical signals along the endothelium.
Methods
Tissue preparation and intracellular recording
Procedures were approved by the Institutional Animal Care and Use Committee and performed in accord with the National Research Council's Guide for the Care and Use of Laboratory Animals (8th Ed. 2011). Male C57BL/6 mice (bred at the University of Missouri; age: 3–6 months; n=45) were anesthetized (pentobarbital; 60 mg/kg intraperitoneal injection. Abdominal muscles were removed bilaterally; feed arteries (superior epigastric) were dissected free from surrounding tissue. Using gentle trituration following mild enzymatic digestion, SMCs were dissociated to produce intact EC tubes (width: 60 μm, length: 1–3 mm).29, 30 Experiments were performed at 32 °C and completed within 3 h.29, 30
A freshly-isolated EC tube was secured at each end in a tissue bath on the stage of an inverted microscope and superfused continuously with physiological salt solution [PSS; composition in mM: 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 N-2-Hydroxyethylpiperazine-m′-2-ethanesulfonic acid (HEPES), 10 Glucose]. A pair of intracellular microelectrodes (borosilicate glass; tip resistance, 150–200 MΩ) coupled to independent amplifiers were inserted into ECs along the midline of the tube with a separation distance of 50–2000 μm. With individual ECs ~35 μm long,29 50 μm separation enabled `local' responses to be recorded from Site 2 as close to the signal origin (Site 1) as possible while ensuring that microelectrodes were in separate (adjacent) cells.30 Successful dual impalements were indicated by correspondence of electrical events between microelectrodes.
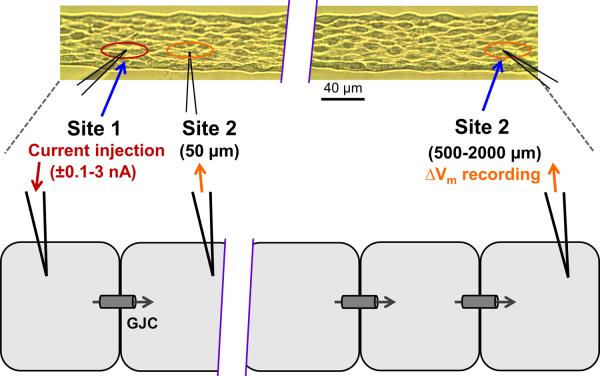
Figure 1 illustrates the experimental approach and summarizes the analyses employed. To study electrical conduction over distance (Figs. 2 and 3), impalement at Site 1 was maintained while the microelectrode for recording Vm at Site 2 was repositioned at the next distance (order randomized across experiments). As standard procedure for evaluating interventions, separation distance was maintained at 500 μm (Figs. 4–8)7, 30 corresponding to ~15 ECs placed end-to-end. Current was microinjected (±0.1–3 nA; 2s pulse) at Site 1 while recording Vm at Site 2 under control conditions, during activation of SKCa/IKCa with NS309, SKA-31, or ACh and during inhibition of SKCa/IKCa with apamin + charybdotoxin (Ap + ChTX). Entire EC tubes were exposed by adding agents to the superfusion solution and Vm was confirmed to be the same between microelectrodes to ensure that the entire EC tube was isopotential before current injections.
Figure 1. Experimental design.
Inset at top: dual intracellular electrodes positioned in an EC tube (Differential Interference Contrast image). Site 1 and Site 2 shown within ovals correspond to cartoon below. Current (±0.1 – 3.0 nA, 2s pulses) was injected at Site 1 while Vm was recorded with separation distances between microelectrodes of 50–2000 μm. To determine the role of SKCa/IKCa in tuning electrical conduction, SKCa/IKCa on all cells were activated (NS309, SKA-31, ACh) or inhibited (apamin + charybdotoxin) by addition of respective agents to the superfusion solution. With no change in axial resistance (ra) to current flow through gap junction channels (GJC), changes in conduction amplitude (CA; ΔVm at Site 2/current injected at Site 1; mV/nA), reflect changes in membrane resistance (rm) associated with activation or inhibition of SKCa/IKCa. Data in Figs. 3, 4, 6–8 reflect CA responses to −1 nA current injection (note that −ΔVm/−1 nA = positive CA value). The Fraction of Control CA (Figs. 4C, F and 8C) was defined as CA during treatment/CA under control conditions at the same 500 μm site. Conduction Efficiency (Fig. 3B) was defined as (CA at X μm) / (CA at 50 μm), where X= 50–2000 μm.
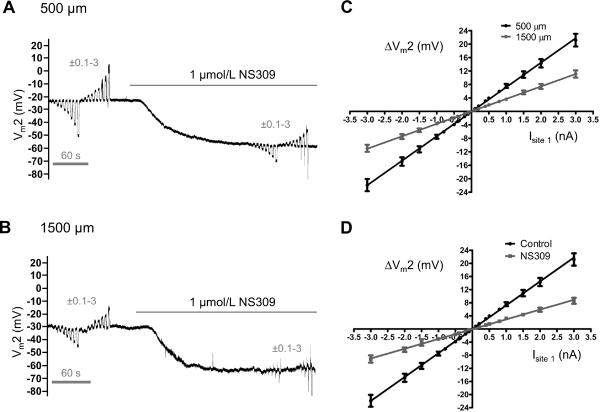
Figure 2. Effect of distance and SKCa/IKCa activation on electrical conduction.
At defined separation distances between intracellular microelectrodes, continuous (paired) recordings at Site 2 during current microinjection at Site 1 were obtained under Control conditions and during NS309 treatment to activate SKCa/IKCa. After each set of paired recordings, washout of NS309 (10–15 min) restored Control Vm before recording at the next distance. A, Representative Vm recording at Site 2 during ±0.1–3 nA microinjected at Site 1 (distance = 500 μm) before and during NS309. From Control Vm of ~−25 mV, NS309 hyperpolarized ECs to ~−60 mV and decreased Vm2 responses. B, As in A with separation distance = 1500 μm. Note reduction in Control Vm2 responses compared to A followed by loss of Vm2 responses during NS309. C, Summary data illustrating the effect of microelectrode separation distance on the change in Vm at Site 2 (ΔVm2) under Control conditions with 500 μm (black line) or 1500 μm (grey line) separation. With reduced slope at greater distance, ΔVm2 responses remained linear through the full range of current injected (R2 = 1). With −1 nA current, absolute ΔVm2 decreased from 7.7 ± 0.6 mV at 500 μm to 3.8 ± 0.3 mV at 1500 μm. D, With separation maintained at 500 μm for the same EC tubes in C, activation of SKCa/IKCa with NS309 (1 μmol/L) reduced CA to 3.1 ± 0.3 mV. Summary data in C and D are means ± S.E.; n = 11.
Note: Panels C and D include (with permission: British Journal of Pharmacology © 2011) control data from 8 experiments presented in Figure 2B,C of Behringer et al.30
Figure 3. Effect of SKCa/IKCa activation on spatial decay of electrical conduction.
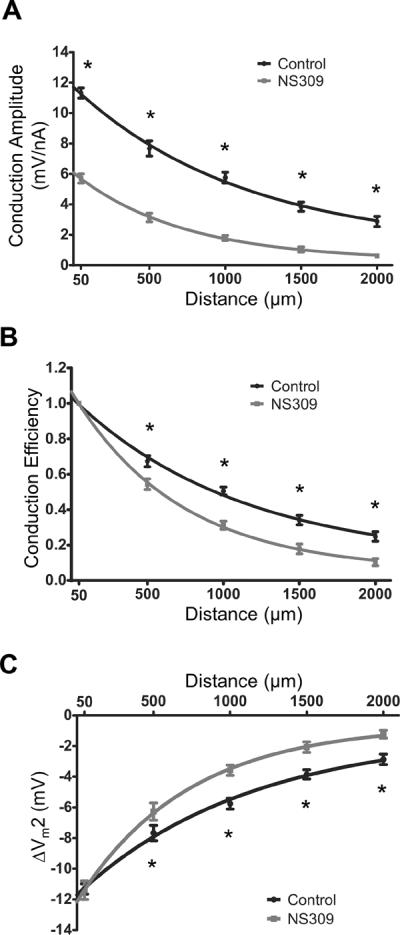
Summary data (means ± S.E.) illustrating electrical conduction versus distance between intracellular microelectrodes before and during treatment with NS309 (1 μmol/L). At each distance, continuous (paired) recordings were obtained under Control conditions and during NS309. A, For Conduction Amplitude (−1 nA microinjected at Site 1), NS309 reduced the local response by half and λ by ~40% (Control: 1380±80 μm; NS309: 850±60 μm). B, Conduction Efficiency = data from A normalized to respective values at 50 μm before and during NS309; note greater decay with NS309. C, With current microinjection adjusted to produce the same local ΔVm (Control: −1 nA; NS309: −2 nA), the ΔVm2 (= resting Vm - peak response Vm) with distance indicates greater decay of hyperpolarization with NS309. For these experiments, n = 11 at 50 – 1,500 μm; n=7 at 2,000 μm). *Control significantly different from NS309, P < 0.05
Note: Panel A includes (with permission: British Journal of Pharmacology © 2011) control data from 8 experiments presented in Figure 2B,C of Behringer et al.30
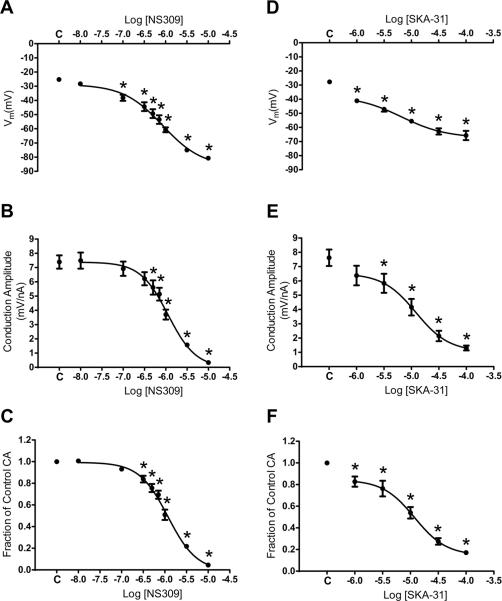
Figure 4. Effect of progressive activation of SKCa/IKCa on electrical conduction.
Summary data (means ± S.E.) for continuous recordings at Site 2 located 500 μm from current microinjection (−1 nA) at Site 1. A, resting Vm with increasing [NS309]; C indicates Control. B, Conduction Amplitude as a function of [NS309]; C indicates Control. C, As in B indicating effect of SKCa/IKCa activation with NS309 relative to Control (Fraction of Control CA = CA during respective [NS309] / Control CA). D, as in A for SKA-31. E, as in B for SKA-31. F, as in C for SKA-31. *Significantly different from Control, P < 0.05, n = 6–8 in each panel. Note reduced potency and efficacy for SKA-31 versus NS309.
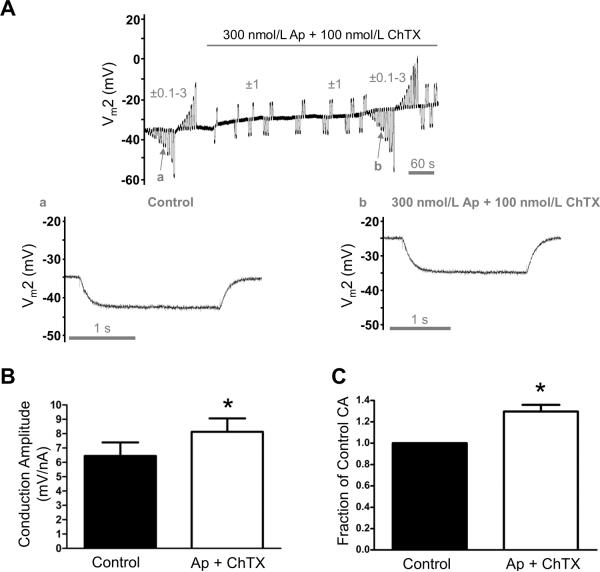
Figure 8. Enhanced electrical conduction during SKCa/IKCa blockade.
All data are from continuous (paired) recordings 500 μm from the site of current microinjection. A, Representative recording indicating Vm2 responses to ±0.1–3 nA microinjections before and during SKCa/IKCa block with Ap (300 nmol/L) + ChTX (100 nmol/L). Note depolarization (~10 mV) and increases in Vm2 responses during Ap + ChTX. a and b correspond to arrows in A and illustrate Vm2 responses to −1 nA injected at Site 1 during Control and subsequent exposure to Ap + ChTX: ΔVm2 was −8 mV in a and −10 mV in b. Records are from EC tube with resting Vm = −34 mV to illustrate depolarization and increased CA associated with blocking SKCa/IKCa. B, Summary data (means ± S.E.) for Control and with Ap + ChTX. C, Data from B expressed as Fraction of Control Conduction Amplitude (= CA during Ap + ChTX /Control CA) to illustrate relative effect of SKCa/IKCa inhibition. *Significantly different from Control, P < 0.05 (n = 6).
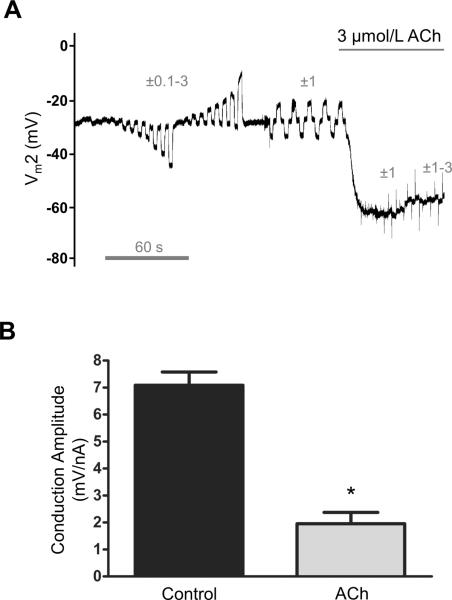
Figure 7. Acetylcholine impairs electrical conduction.
Data are from continuous (paired) recordings of Vm at Site 2 located 500 μm from current microinjection (−1 nA). A, Representative recording illustrating responses to ±0.1–3 nA before and to ±1, 2, and 3 nA during ACh (3 μmol/L). The Vm during ACh was −67 ± 5 mV (n=6). B, Summary data (means ± S.E.; n=6) for Conduction Amplitude (CA). Note decrease in CA during ACh. Hyperpolarization to −2 nA during ACh (−3.9 ± 0.8 mV, n=6) was significantly less (P < 0.05) than hyperpolarization to −1 nA under Control conditions (−7.1 ± 0.5 mV, n=6). *Significantly different from Control, P < 0.05. Following washout of ACh, CA returned to 7.2 ± 0.5 mV/nA (n=6).
Data analyses
One EC tube was studied per mouse. Analyses included: Resting Vm (mV) under Control conditions; Change in Vm (Δ mV) = peak response Vm – preceding baseline Vm; Conduction Amplitude (CA, mV/nA) = Vm at Site 2 / current injected at Site 1; Fraction of Control CA = CA during treatment/preceding control CA; Conduction Efficiency = CA at each distance/CA at 50 μm separation; Length constant (λ) = distance over which the electrical signal decayed to 37% (1/e) of the `local' value. Data were analyzed using Analysis of Variance with Bonferroni post-hoc comparisons, regression analyses, and paired t-tests. Differences were accepted as statistically significant with P < 0.05. Summary data are presented as means ± S.E. Values for n refer to the number of EC tubes studied under respective conditions.
Results
Effects of SKCa/IKCa activation on electrical conduction over distance
Resting Vm was −26±2 mV at Sites 1 and 2 independent of separation distance (n=11). As shown in Figure 2, ΔVm at Site 2 increased in direct proportion to current injected irrespective of polarity. The amplitude of ΔVm2 for each current decreased when separation distance between microelectrodes was increased from 500 to 1500 μm (Figs. 2A–2C) yet the I–V relationship remained linear (Fig. 2C). At 500 μm separation, the slope of the I–V relationship through ± 0.1–3 nA was 7.5±0.5 mV/nA. When calculated for −1 nA, CA (500 μm) was not different (7.7±0.5 mV/nA; n=11), substantiating −1 nA as a standard reference.
The ΔVm (−37 mV) in response to 1 μmol/L NS309 corresponds to the peak hyperpolarization obtained with ACh (3 μmol/L).30 At each distance, opening SKCa/IKCa with 1 μmol/L NS309 reduced CA (Figs. 2A,2B) yet the I–V relationship remained linear (Fig. 2D). Thus NS309 decreased CA at 500 μm to nearly the same extent as did increasing distance to 1500 μm under Control conditions (Fig. 2C). Following continuous paired recordings (Control, NS309) at each distance, washout of NS309 (10–15 min) restored Control Vm and CA (e.g., CA at 500 μm: Control, 7.7 ± 0.4 mV/nA; NS309 washout, 7.9 ± 0.4 mV/nA, n=16), confirming the reversibility of SKCa/IKCa activation. The next separation distance was evaluated in the same manner.
During current injections, NS309 (1 μmol/L) reduced ΔVm2 at the `local' site by half and decreased λ by ~40% (Control: 1380±80 μm; NS309: 850±60 μm; Fig. 3A, P<0.05). By normalizing CA at each separation distance to respective `local' values, Conduction Efficiency was also reduced at each distance (P < 0.05; Fig. 3B). The injection current required for the same local change in Vm obtained with −1 nA under Control conditions increased to −2 nA with 1 μmol/L NS309, consistent with a corresponding decrease in rm. When local hyperpolarization was thereby matched between conditions, the decay in ΔVm2 over distance was again greater (P<0.05) with NS309 (Fig. 3C; Supplemental Table I). Thus, activating SKCa/IKCa impaired electrical conduction irrespective of ΔVm at the site of current injection.
For the following experiments, current was injected at Site 1 while Vm was recorded at Site 2 with 500 μm separation maintained between microelectrodes.7, 30 The stability of impalements enabled multiple treatments to be evaluated during continuous (paired) recordings. Responses were evaluated through the full range of current microinjection with data presented for −1 nA.
Progressive inhibition of electrical conduction during graded activation of SKCa/IKCa
A question central to these experiments was: Can electrical conduction be `tuned' according to the level of SKCa/IKCa activation? Current was injected at Site 1 and Vm recorded at Site 2 (500 μm) before and during increments in SKCa/IKCa activation with recovery between each treatment. Hyperpolarization began at 100 nmol/L NS309 and increased with [NS309] (Fig. 4A; P < 0.05); maximal response at 10 μmol/L corresponded to Vm = −81 ± 1 mV. Impairment of CA; P<0.05) began at 300 nmol/L NS309 and CA decreased as [NS309] increased with conduction abolished at 10 μmol/L (Figs. 4B, 4C). At 1 μmol/L, NS309 reduced CA by half (Figs. 4B, 4C). In separate experiments, using SKA-31 to activate SKCa/IKCa24 produced effects similar to NS309 though with attenuated potency and efficacy (compare Figs. 4D–4F with Figs. 4A–4C). Nevertheless, for both pharmacological agents, a `threshold' Vm associated with significant reduction in CA occurred at ~−40 mV (Figs. 4A, 4D). These findings indicate that the efficacy of electrical conduction along the endothelium can be tuned according to the level of SKCa/IKCa activation.
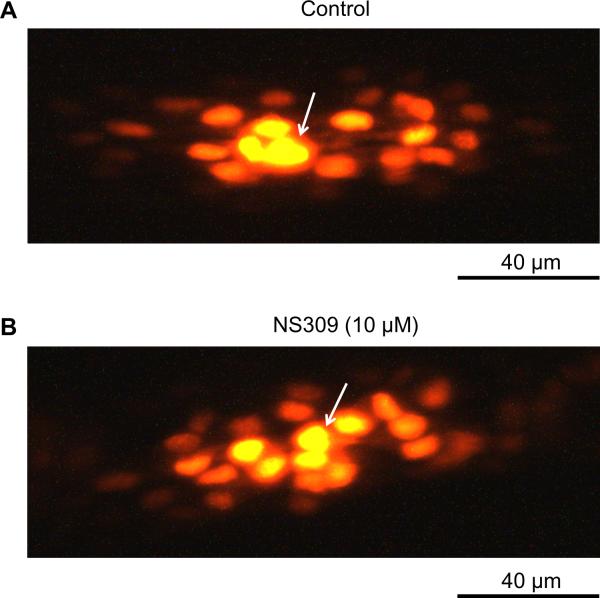
Maintenance of dye transfer during inhibition of electrical conduction
Impairment of electrical conduction during SKCa/IKCa activation is interpreted to reflect greater current dissipation along the endothelium. Thus an essential question is: Does intercellular coupling persist when electrical conduction is inhibited? We used dye transfer as a functional qualitative index of GJC patency between neighboring ECs.4, 7, 10, 27, 30 An EC tube was exposed to the NS309 concentration that inhibited conduction (10 μmol/L) beginning 5 min before and throughout recordings. With 0.1% propidium iodide dye (MW = 668.4 Da) in the microelectrode, fluorescent images were acquired following 30 min of continuous recording. Under Control conditions (Fig. 5A) and during hyperpolarization (to −81 ± 4 mV) with inhibition of electrical conduction [CA (500 μm): 0.3 ± 0.1 mV/nA)], dye microinjected into one EC readily spread to neighboring ECs (Fig. 5B; n=3). Thus dye transfer during maximal activation of SKCa/IKCa with NS309 was qualitatively similar to Control, confirming that intercellular coupling through GJCs remained patent during absence of electrical conduction. For reference, in EC tubes exposed to carbenoxolone (100 μmol/L) or β-glycyrrhetinic acid (40 μmol/L) to block GJCs, dye transfer was inhibited reversibly along with electrical conduction.30
Figure 5. Intercellular dye transfer through GJCs is maintained during SKCa/IKCa activation with NS309.
Propidium iodide dye (0.1% in 2M KCl) was included in microelectrode during intracellular recording to evaluate intercellular coupling through gap junction channels. Arrows indicate impaled cell in each panel and recordings lasted 30 min. A, Dye transfer during Control conditions. B, As in A with NS309 (10 μmol/L) introduced 5 min prior to cell penetration. With 500 μm separation between microelectrodes, cells hyperpolarized to −81 ± 1 mV and electrical conduction was abolished (Figs. 4A–4C) yet robust dye transfer to surrounding cells was maintained. Images are representative of n=3 experiments.
Inhibition of electrical conduction independent of hyperpolarization
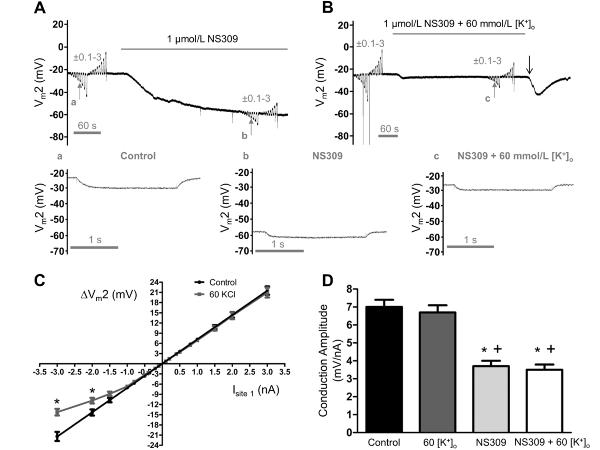
The activation of SKCa/IKCa consistently hyperpolarized ECs, raising the question: Does loss of electrical conduction during SKCa/IKCa activation depend upon hyperpolarization? According to the Nernst relationship, raising [K+]o from 5 (Control) to 60 mmol/L (equimolar replacement of NaCl with KCl) reduces EK from −89 mV to −23 mV and should thereby “clamp” Vm near Control (Vm= −23±1 mV, n=7) and prevent hyperpolarization during K+ channel activation. To test this effect, current was injected and Vm recorded before and during superfusion with either NS309 (1 μmol/L), 60 mmol/L [K+]o, or both together. Despite lack of hyperpolarization, the decrease in CA (500 μm) during NS309 + 60 mmol/L [K+]o was not different from that during NS309 alone (Figs. 6A, 6B). However with 60 mmol/L [K+]o alone the I–V relationship deviated (P<0.05) from linearity when negative current exceeded −1.5 nA (Fig. 6C), attributable to the corresponding reduction in EK. By evaluating Vm2 responses to −1 nA, comparisons between conditions were maintained within the linear range of the I–V relationship. Treatment with NS309 consistently reduced CA (500 μm) by half (Fig. 6D), confirming that inhibition of electrical conduction during SKCa/IKCa activation was independent of a change in Vm.
Figure 6. Impaired electrical conduction during SKCa/IKCa activation is independent of hyperpolarization.
Continuous (paired) recordings of Vm at Site 2 (Vm2) located 500 μm from current microinjections at Site 1. A, Vm2 during ±0.1–3 nA before and during SKCa/IKCa activation with NS309 (1 μmol/L). During NS309, note hyperpolarization (to ~−60 mV) and decrease in Vm2 responses. B, As in A before and during treatment with NS309 + 60 mmol/L [K+]o to prevent hyperpolarization to NS309. Note decrease in Vm2 responses during NS309 similar to findings in A. Transient hyperpolarization near end of recording (“↓”) attributable to slower washout of NS309 versus KCl. a, b and c correspond to small vertical arrows in A and B showing individual recordings of Vm2 during −1 nA injected at Site 1 for (a) control (ΔVm = −6 mV), (b) NS309 (ΔVm = −3 mV), and (c) NS309 + 60 mmol/L [K+]o (ΔVm = −3 mV; note lack of hyperpolarization to NS309 with reduced hyperpolarization to −1 nA current). C, Summary data for ΔVm2 responses to full range of current injection (± 0.1–3 nA) during Control and during 60 mmol/L [K+]o. Note deviation from linearity for ΔV 2 responses to >−1.5 nA during 60 mmol/L [K+]o. *Significantly different from Control, P < 0.05. D, Summary data (means ± S.E.) for CA at Site 2 to −1 nA current injection at Site 1 during Control, during 60 mmol/L [K+]o, during NS309 (1 μmol/L), and during NS309 (1 μmol/L) + 60 mmol/L [K+]o. *Significantly different from Control (P < 0.05). +Significantly different from 60 mmol/L [K+]o, P < 0.05. Summary data (means ± S.E.) in C and D are for n = 7.
Inhibition of conduction by ACh
To determine whether a physiological agonist that activates SKCa/IKCa exerts similar effects, EC tubes were superfused with ACh (3 μmol/L).30 During peak hyperpolarization, responses at Site 2 decreased by ~70% versus Control (Fig. 7). Exposure to ACh + 60 mmol/L [K+]o confirmed that ACh inhibited CA (500 μm) irrespective of hyperpolarization (Supplemental Fig. I).Thus activating SKCa/IKCa with a classic vasodilator also impaired electrical conduction irrespective of hyperpolarization.
Enhanced electrical conduction with SKCa/IKCa blockade
Progressive activation of SKCa/IKCa impaired electrical conduction in a graded manner (Fig. 4). Thus if a subpopulation of SKCa/IKCa were open at rest than inhibition of these channels should enhance electrical conduction. This prediction was tested by injecting current and recording Vm before and during exposure to Ap + ChTX. Exposure to these SKCa/IKCa blockers depolarized ECs (Control: −28±2 mV; Ap + ChTX: −18±2 mV; n=6; P<0.05) and increased CA (500 μm) by ~30% (P<0.05; Fig. 8). Further, the relationship between ΔVm at Site 2 (500 μm) and current injected at Site 1 remained linear (with greater slope) through the entire range of current injection (R2 ≥ 0.99; n = 6).
A summary of the treatment effects of the preceding interventions (Figures 4, 6, 7, 8) on electrical conduction at a standard reference distance (500 μm) is given in Supplemental Table II.
Negligible role for KATP or BKCa channels
If manipulating SKCa/IKCa activity alters electrical conduction than complementary effects would be expected for other K+ channels. We tested for such an effect of ATP-regulated K+ channels (KATP) using levcromakalim (10 μmol/L; n=5). However this KATP opener22, 25 had no significant effect on resting Vm (−27±1 mV) or CA (500 μm; Control, 8.4 ± 1.1, levcromakalim: 8.6 ± 1.1 mV/nA). Complementary experiments investigating a role for the large conductance Ca2+ (and voltage) - sensitive K+ channel (BKCa) using the activator NS161922, 25 (10 or 30 μmol/L; n=4) were also without effect on resting Vm (−27±2 mV) or CA (500 μm; Control, 8.7±1.3, NS1619: 9.3±1.1 mV/nA). These findings indicate that neither KATP nor BKCa are functionally expressed in EC tubes.
Negligible role for nitric oxide
Endothelial cell hyperpolarization may also promote nitric oxide (NO) synthesis.31, 32 We therefore tested whether inhibition of NO synthase altered the effect of SKCa/IKCa activation on electrical conduction. Irrespective of treatment with the inhibitor Nω-nitro-L-arginine (100 μmol/L), exposure to NS309 (1 μmol/L) or to ACh (3 μmol/L) suppressed CA (500 μm) to the same extent (Supplemental Fig. II).
Discussion
This study has revealed the ability of membrane ion channel activation to tune the efficacy of electrical conduction along the endothelium of resistance vessels. Using intact EC tubes freshly isolated from feed arteries of mouse skeletal muscle, findings illustrate that activation of SKCa/IKCa impairs axial signal transmission. This effect is explained by a fall in membrane resistance (rm) that dissipates charge as current flows from cell to cell along the endothelium. During intracellular microinjection of propidium iodide, robust dye transfer between neighboring ECs confirmed that cell-cell coupling through GJCs was maintained in the absence of electrical conduction. Thus, irrespective of SMCs or of changing cell-cell coupling through GJCs, dynamic changes in rm can profoundly alter the efficacy of electrical signal transmission along the endothelium of resistance vessels. Remarkably, such tuning of electrical conduction was governed by ion channels that are insensitive to voltage.
The nature of electrical conduction along EC tubes
Conduction amplitude was defined as the slope of the I–V relationship with responses routinely evaluated at −1 nA; i.e., within the linear range throughout our experiments. At a standard reference distance of 500 μm, our values of CA (~8 mV/nA; Figures 2C–D, 3A) compare favorably with values (~7 mV per 0.8 nA) calculated for the conduction of hyperpolarization along resistance arteries of hamster skeletal muscle.7 Moreover, hyperpolarizing and depolarizing signals conducted with similar efficacy along EC tubes as shown by the linear (i.e., ohmic) changes in Vm throughout the range of currents injected (±0.1–3 nA) at each distance (Fig. 2). Such behavior indicates low functional expression of voltage-sensitive ion channels and is consistent with the lack of effect of NS1619 on Vm or CA. In turn, the deviation from linearity observed during hyperpolarization with >1.5 nA in the presence of 60 mmol/L [K+]o (Fig. 6C) can be explained by the reduction in EK. In contrast to the present findings, depolarizing currents injected into ECs (or SMCs) of feed arteries from hamsters evoked less than half of the absolute change in Vm than did hyperpolarizing current of equal intensity.7 Such deviation observed for intact vessels (versus the linearity of responses for EC tubes) may be attributed to the influence of SMCs through myoendothelial coupling;7, 9 e.g., through voltage gated K+ channels (Kv) that may not otherwise be present in ECs.25
Whereas activating SKCa/IKCa with NS309 (1 μmol/L) reduced CA by 50% or more at each distance (Fig. 3A), comparing absolute values does not resolve changes in the nature of electrical conduction because the local response to current injection (i.e., change in Vm) is reduced. Expressing responses at each distance relative to the local response provides a normalized index of “Conduction Efficiency” and illustrates significantly greater decay in electrical conduction during SKCa/IKCa activation when compared to Control (Fig. 3B). However, it is important to evaluate the effect of SKCa/IKCa activation when conduction is initiated from a similar local change in Vm. We therefore compared responses to −2 nA current during NS309 with responses to −1 nA under Control conditions. With local responses (ΔVm) not different between conditions, the decay in Vm over distance remained significantly greater during SKCa/IKCa activation (Fig. 3C; Supplemental Table I).
Along the entire distance (2000 μm) studied, responses decayed by 4.4% per 100 μm. This value is consistent with ~5% per 100 μm reported for the decay of electrical conduction along the endothelium of retinal arterioles.33 Further, the consistency of such behavior suggests that the biophysical properties of EC tubes determined here can be applied to the endothelium of vessels in other resistance networks. Indeed, λ determined here for electrical conduction (~1.4 mm; Fig. 3) is consistent with control values reported for intact guinea pig arterioles (~1.1 to 1.6 mm)15 and hamster retractor feed arteries (1.2 mm).16 A limitation of the present study is the lack of SMCs, which precludes resolution of how SKCa/IKCa activation may influence electrical signaling through myoendothelial GJCs. Nevertheless, even with SMCs present in earlier studies,15, 16, 33 the similarity in values for λ across preparations and laboratories supports the role of the endothelium as the primary cellular pathway for axial current flow. In turn, minimal charge loss through myoendothelial GJCs is explained by the high input resistance of SMCs.8
Tuning electrical conduction independent of GJC regulation
Electrical conduction along the endothelium is promoted by high rm preventing signal dissipation and low ra through GJCs between neighboring cells. Whereas GJCs have been a focus for regulating vascular conduction,5, 17–21 the present study demonstrates that electrical conduction along the endothelium can be progressively inhibited by graded activation of SKCa/IKCa (Fig. 4). This interpretation is consistent with the robust expression and ionic conductance of both KCa2.3 (SKCa) and KCa3.1 (IKCa) channels estimated from patch clamp studies of native ECs freshly isolated from mouse aortae23 and the established role of SKCa/IKCa in governing EC hyperpolarization.9, 22, 25. From a typical resting Vm of ~−25 mV, incremental activation of SKCa/IKCa with NS309 approached EK and coincided with loss of conduction (Fig. 4A). In a reciprocal manner, blocking SKCa/IKCa with Ap + ChTX depolarized EC tubes by ~10 mV with a ~30% increase in CA (500 μm; Fig. 8). This modest effect of SKCa/IKCa inhibition compared to SKCa/IKCa activation on electrical conduction can be explained by a low percentage of the total SKCa/IKCa in EC membranes being open under Control conditions.
The SKCa/IKCa opener SKA-31 had less potency and reduced efficacy compared to NS309 (Figs. 4A, 4D). Nevertheless, the apparent threshold of channel activation required to significantly reduce CA corresponded to a Vm of ~−40 mV for both agents (0.3 μmol/L NS309, 3 μmol/L SKA-31; Fig. 4). Hyperpolarization may also promote the production of NO31, 32 which may in turn alter rm34 or cell-to-cell coupling through acting on GJCs.35 Nevertheless, the inhibition of electrical conduction by NS309 was maintained when hyperpolarization was prevented (Fig. 6) and when NO synthesis was inhibited (Supplemental Fig. II). Importantly, and similar to the direct activation of SKCa/IKCa by NS309 and SKA-31, CA (500 μm) was reduced ~70% during indirect activation of these channels by ACh (Fig. 7). Further, as seen with NS309, preventing hyperpolarization to ACh (Supplemental Fig. I) or inhibiting NO synthesis (Supplemental Fig. II) did not alter the ability of ACh to suppress electrical conduction. Thus the modulation of rm (and tuning of electrical conduction) along the endothelium may reflect a more generalized response to physiological agonists that signal through G protein-coupled receptors36 irrespective of NO.
The present findings support the hypothesis that opening ion channels in the plasma membrane impairs electrical conduction along the endothelium. As dye transfer remained intact during exposure to NS309 when conduction was abolished (Fig. 4), loss of conduction cannot be attributed to closure of GJCs. In turn, despite the lack of effect for levcromakalim or NS1619, we infer that the activation or inhibition of other ion channels that are functionally expressed in membranes of electrically coupled cells can have effects consistent with those shown here for SKCa/IKCa. Consistent with this inference are recent findings from rat mesenteric arteries, where spreading vasodilation in response to ACh (or isoproterenol) was enhanced when K+ channels (BKCa and Kv) were inhibited in SMCs that were electrically coupled to ECs.37 Hyperpolarization can activate other K+ channels (e.g., inward rectifying, KIR) to increase Vm and enhance electrical conduction.11, 38 Nevertheless, it is unlikely that such a role would be manifest in EC tubes given the linearity of the I–V relationship (Fig. 2) and a resting Vm (~−25mV) above that associated with the negative slope conductance of KIR.25 Further, given the same decrease in CA to NS309 or ACh when hyperpolarization was prevented (Figs. 6D and Supplemental Fig. I, respectively), it appears unlikely that that activation of KIR contributes to electrical conduction along the endothelium. However, this conclusion does not preclude a role for KIR expressed in SMCs to contribute to conducted vasodilation along intact vessels.11, 38
Summary and conclusions
Electrical signaling underlies the correspondence between changes in Vm and diameter along the resistance vasculature.6, 7, 10 Previous studies have focused on the role of intercellular coupling through GJCs in determining the efficacy of electrical conduction,1–3, 8 particularly as it applies to conducted vasodilation along arterioles and ascending vasodilation during exercise.2,14 Paradigms for studying conducted responses have typically involved stimulating a vessel segment (or the tissue in which vessels are embedded) while monitoring vasomotor responses at sites remote from the region of stimulation.14, 18, 19, 21, 37, 39, 40 However there is ambiguity in defining the number of cells activated, the precise stimulus intensity at the signal origin, and the specific role(s) of respective cell layers. In the present study, the endothelium of resistance arteries was isolated as an intact tube to eliminate the influence of blood flow or surrounding cells and tissue. Intracellular microelectrodes enabled electrical conduction to be initiated from a point source (single cell) within the electrical syncytium using prescribed current pulses while monitoring Vm at defined distances. In turn, selective activation (or inhibition) of SKCa/IKCa along the endothelium resolved how electrical conduction can be tuned through changes in membrane resistance while individual cells remain coupled to each other through GJCs.
This study presents two novel findings. First, that electrical conduction can be finely tuned through the activity of voltage-insensitive membrane ion channels. Thus our experiments are the first to demonstrate an integral role for SKCa/IKCa activation in governing EC signaling that is distinct from their established role of initiating EC hyperpolarization9, 24 (Fig. 9). Second, that SKCa/IKCa can define the spatial domain of electrical signaling along the endothelium (Fig. 9). The SKCa/IKCa have gained recognition as therapeutic targets in light of their ability to initiate hyperpolarization and promote vasodilation to increase tissue blood flow.24, 41, 42 The present findings imply that selectively targeting ion channels for therapeutic intervention should account for integrated effects throughout resistance networks as well as local effects on vasomotor tone.
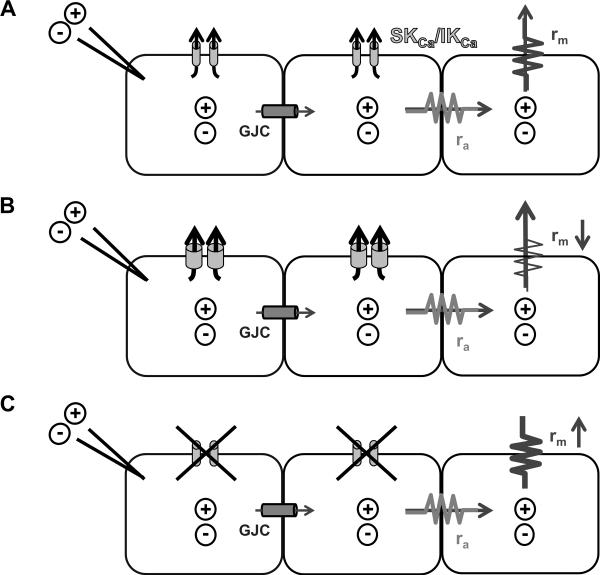
Figure 9. Role for SKCa/IKCa in tuning electrical conduction along EC tubes.
Cartoon illustrating biophysical determinants of electrical signaling along neighboring cells in an EC tube. A, Electrical current (+/-) microinjected into one cell spreads to neighboring cells through gap junction channels (GJC). B, Activation of SKCa/IKCa (e.g., with NS309, SKA-31 or ACh; Figs. 3, 4, 7) increases current leak through the plasma membrane (i.e., rm decreases), thus less current is available to spread to neighboring cells through GJC. C, Blocking SKCa/IKCa (e.g., with apamin + charybdotoxin; Fig. 8) reduces current leak across the plasma membrane (i.e., rm increases) thus more current is available to spread to neighboring cells through GJC. Axial resistance to current flow between cells through GJC (ra) is assumed to remain constant. With respective agents superfused over the entire EC tube, SKCa/IKCa channels were either opened (e.g. via NS309; B) or closed (Ap + ChTX; C) along the entire EC tube.
Supplementary Material
Novelty and Significance.
What is known?
The endothelium conducts electrical signals along resistance vessels to coordinate relaxation of smooth muscle cells.
Gap junctions provide a low-resistance pathway for current to flow between endothelial cells.
Small- and intermediate- Ca2+-activated K+ channels (SKCa/IKCa) are highly expressed in endothelial cells and initiate hyperpolarization.
What new information does this article contribute?
Electrical conduction of hyperpolarization and depolarization along the endothelium produce equivalent (but opposite) changes in membrane potential.
Irrespective of gap junctions or charge polarity, opening voltage-insensitive ion channels dissipates electrical signals along the endothelium.
Activation of SKCa/IKCa channels effectively “tunes” the ability of the endothelium to conduct electrical signals.
The endothelium is recognized as the principal pathway for initiating vasodilation through SKCa/IKCa activation and for the conduction of hyperpolarization in resistance networks. In regulating conducted vasodilation, attention has focused on the role and manipulation of intercellular gap junctions to alter the resistance to current flow between cells. In contrast, we studied whether altering the electrical resistance of endothelial plasma membranes via activation or blockade of ion channels can regulate electrical conduction.. Our new findings demonstrate that, irrespective of charge polarity, electrical conduction along the endothelium is impaired during SKCa/IKCa activation and is enhanced by SKCa/IKCa blockade. At the same time, neighboring cells remain well coupled to each other through gap junctions. We suggest that impaired tissue blood flow during endothelial dysfunction may reflect enhanced ion channel activation (i.e. “leaky” plasma membranes) that impairs coordinated control of vascular resistance. Therapeutic interventions designed to improve tissue blood flow by altering ion channel activation should take into account the effects on electrical conduction in resistance networks.
Acknowledgements
Dr. William F. Jackson provided helpful comments during the initial stages of this study.
Sources of Funding: This work was supported by NIH grants R37-HL041026, R01-HL086483 and F32-HL110701.
Non-standard Abbreviations and Acronyms
- Ap
apamin
- ACh
acetylcholine
- BKCa
large conductance Ca2+ activated K+ channels
- CA
conduction amplitude
- ChTX
charybdotoxin
- EC
endothelial cell
- SKCa/IKCa
small- and intermediate-conductance Ca2+ activated K+ channels
- KATP
ATP-regulated K+ channels
- KIR
inward-rectifying K+ channels
- Kv
voltage-gated K+ channels
- NO
nitric oxide
- NS1619
1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-2H-benzimidazol-2-one
- NS309
6,7-dichloro-1H-indole-2,3-dione 3-oxime
- SMC
smooth muscle cell
- SKA-31
naptho[1,2-d]thiazol-2-ylamine
- Vm
membrane potential
Footnotes
Subject Codes: [95] Endothelium/vascular type/nitric oxide, [106] Electrophysiology, [118] Cardiovascular Pharmacology, [138] Cell signaling/signal transduction, [152] Ion channels/membrane transport
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beny JL. Information networks in the arterial wall. News Physiol Sci. 1999;14:68–73. doi: 10.1152/physiologyonline.1999.14.2.68. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt VJ, Wolfle SE, Boettcher M, de Wit C. Gap junctions synchronize vascular tone within the microcirculation. Pharmacol Rep. 2008;60:68–74. [PubMed] [Google Scholar]
- 3.Bagher P, Segal SS. Regulation of blood flow in the microcirculation: Role of conducted vasodilation. Acta Physiol (Oxf) 2011;202:271–284. doi: 10.1111/j.1748-1716.2010.02244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emerson GG, Segal SS. Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ Res. 2000;86:94–100. doi: 10.1161/01.res.86.1.94. [DOI] [PubMed] [Google Scholar]
- 5.Looft-Wilson RC, Payne GW, Segal S. Connexin expression and conducted vasodilation along arteriolar endothelium in mouse skeletal muscle. J Appl Physiol. 2004;97:1152–1158. doi: 10.1152/japplphysiol.00133.2004. [DOI] [PubMed] [Google Scholar]
- 6.Wolfle SE, Schmidt VJ, Hoyer J, Kohler R, de Wit C. Prominent role of KCa3.1 in endothelium-derived hyperpolarizing factor-type dilations and conducted responses in the microcirculation in vivo. Cardiovasc Res. 2009;82:476–483. doi: 10.1093/cvr/cvp060. [DOI] [PubMed] [Google Scholar]
- 7.Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: Role in vasomotor control. Circ Res. 2000;87:474–479. doi: 10.1161/01.res.87.6.474. [DOI] [PubMed] [Google Scholar]
- 8.Diep HK, Vigmond EJ, Segal SS, Welsh DG. Defining electrical communication in skeletal muscle resistance arteries: A computational approach. J Physiol. 2005;568:267–281. doi: 10.1113/jphysiol.2005.090233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garland CJ, Hiley CR, Dora KA. EDHF: Spreading the influence of the endothelium. Br J Pharmacol. 2011;164:839–852. doi: 10.1111/j.1476-5381.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welsh DG, Segal SS. Endothelial and smooth muscle cell conduction in arterioles controlling blood flow. Am J Physiol. 1998;274:H178–186. doi: 10.1152/ajpheart.1998.274.1.H178. [DOI] [PubMed] [Google Scholar]
- 11.Crane GJ, Neild TO, Segal SS. Contribution of active membrane processes to conducted hyperpolarization in arterioles of hamster cheek pouch. Microcirculation. 2004;11:425–433. doi: 10.1080/10739680490457836. [DOI] [PubMed] [Google Scholar]
- 12.Figueroa XF, Duling BR. Dissection of two Cx37-independent conducted vasodilator mechanisms by deletion of Cx40: Electrotonic versus regenerative conduction. Am J Physiol Heart Circ Physiol. 2008;295:H2001–2007. doi: 10.1152/ajpheart.00063.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Wit C. Different pathways with distinct properties conduct dilations in the microcirculation in vivo. Cardiovasc Res. 2009;85:604–613. doi: 10.1093/cvr/cvp340. [DOI] [PubMed] [Google Scholar]
- 14.Segal SS, Jacobs TL. Role for endothelial cell conduction in ascending vasodilatation and exercise hyperaemia in hamster skeletal muscle. J Physiol. 2001;536:937–946. doi: 10.1111/j.1469-7793.2001.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirst GD, Neild TO. An analysis of excitatory junctional potentials recorded from arterioles. J Physiol. 1978;280:87–104. doi: 10.1113/jphysiol.1978.sp012374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emerson GG, Neild TO, Segal SS. Conduction of hyperpolarization along hamster feed arteries: Augmentation by acetylcholine. Am J Physiol Heart Circ Physiol. 2002;283:H102–109. doi: 10.1152/ajpheart.00038.2002. [DOI] [PubMed] [Google Scholar]
- 17.Segal SS, Duling BR. Conduction of vasomotor responses in arterioles: A role for cell-to-cell coupling? Am J Physiol Heart Circ Physiol. 1989;256:H838–845. doi: 10.1152/ajpheart.1989.256.3.H838. [DOI] [PubMed] [Google Scholar]
- 18.de Wit C, Roos F, Bolz SS, Kirchhoff S, Kruger O, Willecke K, Pohl U. Impaired conduction of vasodilation along arterioles in Connexin40-deficient mice. Circ Res. 2000;86:649–655. doi: 10.1161/01.res.86.6.649. [DOI] [PubMed] [Google Scholar]
- 19.Figueroa XF, Paul DL, Simon AM, Goodenough DA, Day KH, Damon DN, Duling BR. Central role of Connexin40 in the propagation of electrically activated vasodilation in mouse cremasteric arterioles in vivo. Circ Res. 2003;92:793–800. doi: 10.1161/01.RES.0000065918.90271.9A. [DOI] [PubMed] [Google Scholar]
- 20.Kurjiaka DT, Bender SB, Nye DD, Wiehler WB, Welsh DG. Hypertension attenuates cell-to-cell communication in hamster retractor muscle feed arteries. Am J Physiol Heart Circ Physiol. 2005;288:H861–870. doi: 10.1152/ajpheart.00729.2004. [DOI] [PubMed] [Google Scholar]
- 21.Wolfle SE, Schmidt VJ, Hoepfl B, Gebert A, Alcolea S, Gros D, de Wit C. Connexin45 cannot replace the function of Connexin40 in conducting endothelium-dependent dilations along arterioles. Circ Res. 2007;101:1292–1299. doi: 10.1161/CIRCRESAHA.107.163279. [DOI] [PubMed] [Google Scholar]
- 22.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81:1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- 23.Ledoux J, Bonev AD, Nelson MT. Ca2+-activated K+ channels in murine endothelial cells: Block by intracellular calcium and magnesium. J Gen Physiol. 2008;131:125–135. doi: 10.1085/jgp.200709875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damkjaer M, Nielsen G, Bodendiek S, Staehr M, Gramsbergen JB, de Wit C, Jensen BL, Simonsen U, Bie P, Wulff H, Kohler R. Pharmacological activation of KCa3.1/KCa2.3 channels produces endothelial hyperpolarization and lowers blood pressure in conscious dogs. Br J Pharmacol. 2012;165:223–234. doi: 10.1111/j.1476-5381.2011.01546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation. 2005;12:113–127. doi: 10.1080/10739680590896072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolfle SE, Chaston DJ, Goto K, Sandow SL, Edwards FR, Hill CE. Non-linear relationship between hyperpolarisation and relaxation enables long distance propagation of vasodilatation. J Physiol. 2011;589:2607–2623. doi: 10.1113/jphysiol.2010.202580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emerson GG, Segal SS. Electrical activation of endothelium evokes vasodilation and hyperpolarization along hamster feed arteries. Am J Physiol Heart Circ Physiol. 2001;280:H160–167. doi: 10.1152/ajpheart.2001.280.1.H160. [DOI] [PubMed] [Google Scholar]
- 28.Figueroa XF, Chen CC, Campbell KP, Damon DN, Day KH, Ramos S, Duling BR. Are voltage-dependent ion channels involved in the endothelial cell control of vasomotor tone? Am J Physiol Heart Circ Physiol. 2007;293:H1371–1383. doi: 10.1152/ajpheart.01368.2006. [DOI] [PubMed] [Google Scholar]
- 29.Socha MJ, Hakim CH, Jackson WF, Segal SS. Temperature effects on morphological integrity and Ca2+ signaling in freshly isolated murine feed artery endothelial cell tubes. Am J Physiol Heart Circ Physiol. 2011;301:H773–783. doi: 10.1152/ajpheart.00214.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Behringer EJ, Socha MJ, Polo-Parada L, Segal SS. Electrical conduction along endothelial cell tubes from mouse feed arteries: Confounding actions of glycyrrhetinic acid derivatives. Br J Pharmacol. 2012;166:774–787. doi: 10.1111/j.1476-5381.2011.01814.x. doi:10.1111/j.1476-5381.2011.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stankevicius E, Lopez-Valverde V, Rivera L, Hughes AD, Mulvany MJ, Simonsen U. Combination of Ca2+ -activated K+ channel blockers inhibits acetylcholine-evoked nitric oxide release in rat superior mesenteric artery. Br J Pharmacol. 2006;149:560–572. doi: 10.1038/sj.bjp.0706886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheng JZ, Ella S, Davis MJ, Hill MA, Braun AP. Openers of SKCa and IKCa channels enhance agonist-evoked endothelial nitric oxide synthesis and arteriolar vasodilation. FASEB J. 2009;23:1138–1145. doi: 10.1096/fj.08-120451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang T, Wu DM, Xu GZ, Puro DG. The electrotonic architecture of the retinal microvasculature: Modulation by angiotensin ii. J Physiol. 2011;589:2383–2399. doi: 10.1113/jphysiol.2010.202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yajima K, Nishiyama M, Yamamoto Y, Suzuki H. Inhibition of endothelium-dependent hyperpolarization by endothelial prostanoids in guinea-pig coronary artery. Br J Pharmacol. 1999;126:1–10. doi: 10.1038/sj.bjp.0702254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blasits S, Maune S, Santos-Sacchi J. Nitric oxide uncouples gap junctions of supporting deiters cells from Corti's organ. Pflugers Arch. 2000;440:710–712. doi: 10.1007/s004240000355. [DOI] [PubMed] [Google Scholar]
- 36.Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl 1):S1–324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beleznai TZ, Yarova PL, Yuill KH, Dora KA. Smooth muscle Ca2+ -activated and voltage-gated K+ channels modulate conducted dilation in rat isolated small mesenteric arteries. Microcirculation. 2011;18:487–500. doi: 10.1111/j.1549-8719.2011.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jantzi MC, Brett SE, Jackson WF, Corteling R, Vigmond EJ, Welsh DG. Inward rectifying potassium channels facilitate cell-to-cell communication in hamster retractor muscle feed arteries. Am J Physiol Heart Circ Physiol. 2006;291:H1319–1328. doi: 10.1152/ajpheart.00217.2006. [DOI] [PubMed] [Google Scholar]
- 39.Duling BR, Berne RM. Propagated vasodilation in the microcirculation of the hamster cheek pouch. Circ Res. 1970;26:163–170. doi: 10.1161/01.res.26.2.163. [DOI] [PubMed] [Google Scholar]
- 40.Berg BR, Cohen KD, Sarelius IH. Direct coupling between blood flow and metabolism at the capillary level in striated muscle. Am J Physiol Heart Circ Physiol. 1997;272:H2693–2700. doi: 10.1152/ajpheart.1997.272.6.H2693. [DOI] [PubMed] [Google Scholar]
- 41.Feletou M. Calcium-activated potassium channels and endothelial dysfunction: Therapeutic options? Br J Pharmacol. 2009;156:545–562. doi: 10.1111/j.1476-5381.2009.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dalsgaard T, Kroigaard C, Simonsen U. Calcium-activated potassium channels - a therapeutic target for modulating nitric oxide in cardiovascular disease? Expert Opin Ther Targets. 2010;14:825–837. doi: 10.1517/14728222.2010.500616. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.