Abstract
Dorsal root ganglion (DRG) neurons with dichotomizing axons have been reported in several species and are thought to be related to referred pain. However, these neurons, which have dichotomizing axons to the lumbar muscles and to the knee, have not been investigated. Clinically, pain from the lumbar muscles is sometimes referred to the lower extremities. Two kinds of neurotracers [1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate (DiI) and fluoro-gold (FG)] were used in the present double-labelling study. DiI crystals were placed in the left lower back muscle, and FG was applied to the medial side of the knee. Bilateral DRGs from L1 through L6 were immunoreacted with calcitonin gene-related peptide (CGRP) antibodies and observed under a fluorescence microscope. DRG neurons double-labelled with DiI and FG were recognized only in the ipsilateral DRGs from levels L1 to L6. Approximately 1% of DRG neurons innervating the low back muscles had other axons to the medial side of the knee. In double-labelled neurons, the ratio of CGRP-immunoreactive DRG neurons was 60%. This finding provides a possible neuroanatomical explanation for referred knee pain from the lower back since CGRP is a marker of sensory neurons typically involved with pain perception. However, these neurons are rare, and mechanisms of referred pain may be explained by the convergence–projection hypothesis.
Keywords: Axons, Fluorescent dyes, Low back pain, Knee, Calcitonin gene-related peptide
Introduction
Many studies have reported that lumbar muscles, intervertebral discs, and facet joints are a source of low back pain [5, 17, 19, 36]. Occasionally, pain from these structures is referred to the lower extremities, as far as the area distal to the knee, as leg pain can be elicited by injection of contrast medium or hypertonic saline into the structures described [6, 13, 14, 19].
With a double fluorescent labelling technique, dorsal root ganglion (DRG) neurons with dichotomizing axons projecting to two different peripheral nerves have been found in divergent body areas in different species [25, 29, 34, 35]. Dichotomizing fibres have also been demonstrated electrophysiologically in the peripheral nerves [10, 24]. Thus, dichotomizing nerve fibres of sensory neurons are considered to be a possible substratum of referred pain [1, 32].
DRG neurons with dichotomizing axons have been investigated mostly in visceral organs [25, 29, 34, 35]. Recently, it has been reported that, in rats, approximately 3% of DRG neurons innervating the lumbar facet joints have dichotomized axons projecting to the sciatic nerve [28]. However, no dichotomizing axons projecting to the facet joint and the skin have been observed, which suggests that collateral axons of the DRG neurons to the sciatic nerve terminate in tissues other than the skin, such as muscles or bones. Furthermore, the primary sensory neurons examined included both large and small proprioceptive neurons [28].
CGRP is a marker of sensory neurons typically involved in pain perception [11, 16]. It has been reported that CGRP-immunoreactive (ir) nerve fibres are present within the lumbar muscle, intervertebral discs, and facet joints [2, 3, 4, 33]. There have also been reports of the presence of CGRP-ir DRG neurons innervating the lumbar intervertebral discs and facet joints [20, 23].
The purpose of the present study was to investigate and characterize DRG neurons with dichotomizing axons projecting to both the lumbar muscles and the knee in rats using double and triple fluorescent labelling techniques.
Materials and methods
The studies described in this report were authorized by the appropriate local Ethics or Animal Experimentation Committees of Chiba University. Ten male Sprague-Dawley (SD) rats weighing 250–300 g were used. They were anaesthetized with sodium pentobarbital (40 mg/kg, i.p.) and treated aseptically throughout the experiments. A midline dorsal longitudinal incision was made over the lumbar spine. The left multifidus muscles at the L4 level were exposed under a microscope [9]. A 26-gauge needle with a tip filled with 1 mg of crystals of 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate (DiI) (Molecular Probes, Inc., Eugene, Ore.) was advanced into the muscles. The fascia and skin were then closed. Next, a medial incision was made over the left knee, and a 26-gauge needle with a tip containing two fluoro-gold (FG; Fluorochrome, Denver, Colo.) crystals was advanced into the medial muscles (vastus medialis, gracilis and semimembranosus) and outside of the capsule [9]. The skin was then closed.
Twelve days after surgery, these ten rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.) and perfused transcardially with 0.9% saline, followed by 500 ml of 4% paraformaldehyde in phosphate buffer (0.1 M, pH 7.4). Bilateral DRGs from levels L1 to L6 were resected. The specimens were immersed in the same fixative solution overnight at 4 °C. After storing in 0.01 M phosphate buffer saline (PBS) containing 20% sucrose for 20 h at 4 °C each DRG was sectioned at a thickness of 40 μm on a cryostat. We analysed all the sections that were obtained from each DRG. The number of sections was not always the same from each DRG, but it was always around 30.
Immunohistochemistry of CGRP
Sections of DRGs were collected in PBS. We used the same section to visualize two fluorescence markers and the immunohistochemistry of CGRP. Endogenous tissue peroxidase activity was quenched by soaking the sections for 30 min in 0.3% hydrogen peroxide solution in 0.01 M PBS. Then the specimens were treated for 90 min in blocking solution, 0.01 M PBS containing 0.3% Triton X-100 and 1% normal goat serum, at room temperature. They were processed for CGRP immunohistochemistry with a free-floating ABC technique using rabbit antibody for CGRP (1:2000; Chemicon, Temecula, Calif.) diluted with a blocking solution for 20 h at 4 °C, biotinylated goat anti-rabbit IgG (1:100; Vector Labs, Burlingame, Calif.), and fluorescein isothiocyanate (FITC) avidin D (1:100; Vector Labs, Burlingame, Calif.). After each step, the sections were rinsed three times in 0.01 M PBS. The sections were observed under a fluorescence microscope. DiI- or FG-labelled neurons and neurons double-labelled with DiI and FG (DiI/FG) were counted. In these double-labelled neurons, the ratio of the CGRP-ir neurons was counted. The cross-sectional areas of cell profiles of double-labelled neurons and double-labelled CGRP-ir neurons were also measured with a computer-assisted imaging analysis system (NIH Image software).
Statistical analysis
The data were compared using the non-paired Welch’s t-test. A P value of less than 0.05 was considered statistically significant.
Results
DiI- or FG-labelled DRG neurons
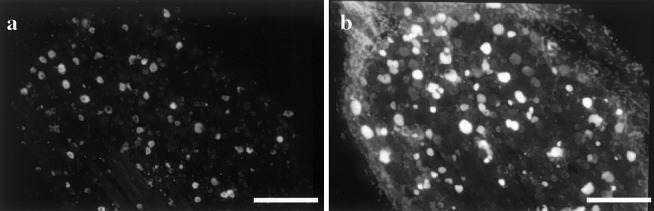
In DiI-labelled DRG neurons transported from the lumbar muscles, labelled neurons were present in the ipsilateral DRGs from L1 through L6 (Fig. 1a, Table 1). No labelled neurons were present in the contralateral DRGs from L1 through L6. Of the DiI-labelled neurons, 70% were recognized in the L2 and L3 DRGs, and the remaining 30% were seen in the L1, L4, L5 and L6 DRGs. Neurons labelled with FG transported from the medial portion of the knee were distributed in the L1–L5 DRGs; 90% were recognized in the L2, L3 and L4 DRGs (Fig. 1b, Table 1).
Fig. 1.
DiI-labelled neurons innervating the lumbar muscles (a) and FG-labelled neurons innervating the medial portion of the left knee (b) in the L4 DRG. Bar=500 μm
Table 1.
Average number of Dil-labelled, FG-labelled, Dil/FG-labelled neurons and Dil/FG-labelled CGRP-ir neurons (n=10)
| Level of DRGs | Dil-labelled neurons | FG-labelled neurons | Dil/FG (double)-labelled neurons | Dil/FG-labelled CGRP-ir neurons |
|---|---|---|---|---|
| L1 | 300±50 | 70±10 | 0.8±0.1 (0.3%*, 1.2%**) | 0.3±0.1 (38%***) |
| L2 | 550±64 | 254±33 | 3.2±0.2 (0.6%, 1.3%) | 2±0.3 (63%) |
| L3 | 577±60 | 524±62 | 8.1±0.9 (1.4%, 1.5%) | 5±0.7 (62%) |
| L4 | 155±18 | 175±21 | 1.8±0.2 (1.2%, 1.0%) | 1.0±0.1 (56%) |
| L5 | 30±5 | 21±5 | 0±0 (0%, 0%) | 0±0 (0%) |
| L6 | 21±5 | 3±0 | 0.3±0.04 (1.4%, 10%) | 0.2±0.3 (67%) |
| Total | 1634±214 | 1047±129 | 14.2±1.8 (0.87%, 1.34%) | 8.5±1.0 (60%) |
* Proportion of Dil/FG-labelled neurons among Dil-labelled neurons
** Proportion Dil/FG-labelled neurons among FG-labelled neurons
*** Proportion of double-labelled CGRP-ir neurons among double-labelled neurons
Neurons double-labelled with DiI and FG (DiI/FG)
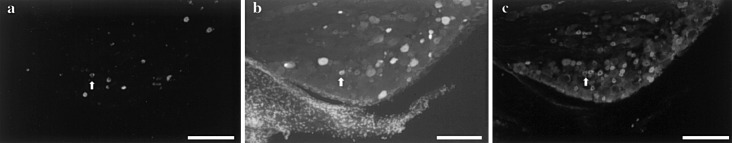
Double-labelled neurons were present in the ipsilateral L1, L2, L3, L4 and L6 DRGs (Fig. 2a, b, Table 1). Among DiI-labelled neurons, the proportion of double-labelled neurons in DRGs was 0.9% and among FG-labelled neurons, the proportion of double-labelled neurons was 1.3% (Table 1). The proportion of double-labelled neurons was significantly less than among DiI- or FG-labelled neurons (P<0.01). There were no significant differences in proportions of double-labelled neurons between the L2, L3 and L4 DRGs (Table 1).
Fig. 2.
DiI-labelled neurons innervating the lumbar muscles (a) and FG-labelled neurons innervating the medial portion of the left knee (b) in the L3 DRG. CGRP immunoreactivity is shown in c Arrow indicates a double-labelled, CGRP-ir neuron. Bar=500 μm
CGRP-ir neurons double-labelled with DiI and FG
CGRP-ir double-labelled neurons with DiI/FG were present in the ipsilateral L1, L2, L3, L4 and L6 DRGs (Fig. 2c, Table 1). Among neurons double-labelled with DiI/FG, CGRP-ir neurons made up 60% (Table 1). The proportions of double-labelled CGRP-ir neurons were not significantly different from those of double-labelled non-CGRP-ir neurons (P<0.05).
Cross-sectional area of cell profiles
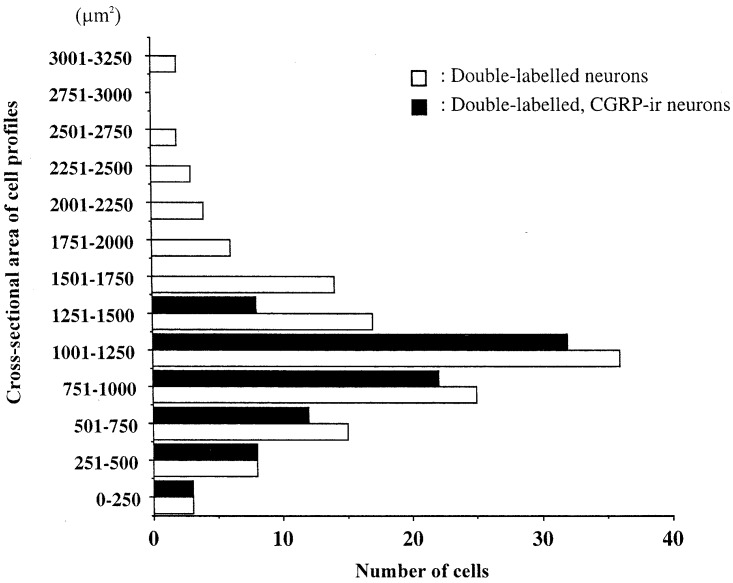
We measured a total of 135 neurons from L1–L6 DRGs from the ten rats we used. The cross-sectional areas of cell profiles of DiI/FG-labelled neurons ranged from 150 μm2 to 3520 μm2. Of the DiI/FG-labelled neurons, CGRP-ir neurons ranged from 150 μm2 to 1285 μm2. The mean cell profile of DiI/FG-labelled CGRP-ir neurons was 775±50 μm2 (mean±SE) (Fig. 3). CGRP immunoreactivity was mainly observed in small neurons.
Fig. 3.
Histograms showing the distribution of size (cross-sectional area) of double-labelled (white columns, n=135) and double-labelled, CGRP-ir neurons (black columns, n=85) in L1–L6 DRGs
Discussion
Many investigators have reported that lesions of the lumbar muscles, intervertebral discs, and facet joints cause pain not only in the lower back but also in the leg [6, 13, 14, 19]. Injection of hypertonic saline into the intervertebral disc or facet joint produces referred pain in the entire leg and foot, which is resolved by injection of lignocaine (lidocaine) into the intervertebral disc or facet joint [17, 19]. These facts indicate that lower back and leg pain can be referred from these structures.
Many muscle pain models in human have been used. Injection of hypertonic saline is a well-established method of inducing experimental local and referred muscle pain [12, 13]. Intraneural microstimulation of nociceptive afferent fibres in muscle tissue has been described and various muscle pain phenomena have been studied using this method [31]. It has been reported that local and referred muscle pain can be elicited by intramuscular electrical stimulation, and indicated that temporal and spatial summation may be involved in eliciting referred muscle pain [7, 8, 15].
Neural mechanisms of referred pain have not yet been clarified, although two hypotheses have been proposed. Firstly, the convergence–projection hypothesis proposed by Ruch suggests that somatic and visceral fibres from each of the primary neurons converge upon one dorsal horn neuron in the spinal cord, and several experimental studies have supported the authenticity of this hypothesis [26, 27, 30]. Secondly, Sinclair’s concept of referred pain suggests that there are primary afferent fibres bifurcating into somatic and visceral structures [32]. Referred pain has been studied both in animal models and humans. However, in this study, we used rats since the structure of the low back and knee joint in rat is similar to that in humans [9].
In DiI-labelled DRG neurons transported from the lumbar muscles, labelled neurons were present in the ipsilateral DRGs from L1 to L6. According to a previous report, the rat lumbar intervertebral disc is innervated by multi-segmental and bilateral dorsal root ganglia [21], whereas the cervical and lumbar facet joints are innervated by ipsilateral DRGs [22, 33]. In the present study, the left lumbar back muscle and the left knee were innervated only by left DRGs. These findings lead us to believe that the lumbar structure and knee may have only ipsilateral sensory innervation.
Approximately 3% of DRG neurons innervating the lumbar facet joints have dichotomized axons projecting to the sciatic nerve [28]. Therefore, double-labelled neurons may cause referred leg pain originating from the facet joint through the sciatic nerve [28]. Interestingly, the primary sensory neurons examined included both large and small neurons transmitting position sense. In the present study, approximately 1% of DRG neurons innervating the low back muscles have dichotomized axons projecting to the medial portion of the knee. DiI-labelled neurons or FG-labelled neurons in L5 and L6 DRGs were rare. Therefore, the proportion of double-labelled neurons in L5 is 0% and that in L6 is 0.3%. In the 1% of DRG neurons innervating the low back muscles and having dichotomized axons projecting to the medial portion of the knee, the proportion of the CGRP-ir neurons that represent pain transmission was 60%. CGRP-ir neurons are distributed among DRG neurons of various sizes. However, the CGRP-ir DRG neurons innervating the lower back muscles have dichotomized axons to the knee that are distributed only among small and intermediate-sized neurons. A study of electrophysiologically characterized neurons in lumbar dorsal root ganglia using dye injection revealed CGRP-like immunoreactivity in 46% of C-fibre neurons, 33% of A δ-fibre neurons, and 17% of A α/β-fibre neurons [18]. These data indicate that CGRP is mainly a marker of pain perception, although CGRP may also be a marker of proprioception. In the present study, double-labelled CGRP-ir neurons innervating the lumbar muscle and knee were only small neurons. We did not examine the electrophysiologically characterized neurons precisely; however, anatomical measurement of cell size leads us to conclude that most CGRP-ir DRG neurons transmit pain sensation, not position.
Pain provoked by the irritation of deep tissues has a characteristic quality and tends to be diffuse. Distribution of pain from the lumbar muscle has been reported to be referred to the thigh and calf [6, 13, 14]. This clinical event is believed to be partly caused by the dichotomizing axons, but as these neurons are rare, it cannot be explained by their existence alone. Graven-Nielsen et al. showed that referred anterior ankle pain developed during saline infusion into the anterior tibial muscle [7]. They observed that the referred pain was delayed compared to the local muscle pain and excluded the possibility that the referred pain can be explained by the simple bifurcation of sensory neurons. They also denied that the convergence of afferent sensory neurons onto single projection neurons could have differential effects on the sensation in the referred area. They concluded that referred pain was due to sensitization of projection neurons, so that normally noxious input may drive the projection neuron to fire at an intensity and frequency normally associated with pain. Thus, our present finding provides, in part, a possible neuroanatomical explanation for pain referred to the knee from the low back. However, part of the mechanism of referred pain may be explained by the convergence–projection hypothesis or by sensitization of projection neurons.
In conclusion, many DRG neurons innervated the lumbar muscle and medial portion of the knee. In DRG neurons only 1% of DRG neurons innervating the low back muscles had other axons projecting to the medial side of the knee. Half of the DRG neurons innervating both areas contained CGRP, a neuropeptide associated with pain transmission. This finding provides a possible neuroanatomical explanation for referred knee pain from the lower back. However, these neurons are rare, and mechanisms of referred pain may be explained by the convergence–projection hypothesis.
References
- 1.Alles Brain Res. 1985;342:382. doi: 10.1016/0006-8993(85)91142-4. [DOI] [PubMed] [Google Scholar]
- 2.Ashton J Orthop Res. 1992;10:72. doi: 10.1002/jor.1100100109. [DOI] [PubMed] [Google Scholar]
- 3.Ashton J Orthop Res. 1994;12:186. doi: 10.1002/jor.1100120206. [DOI] [PubMed] [Google Scholar]
- 4.Beaman Spine. 1993;18:1044. doi: 10.1097/00007632-199306150-00014. [DOI] [PubMed] [Google Scholar]
- 5.Fairbank Spine. 1981;6:598. doi: 10.1097/00007632-198111000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Feinstein B, Langton JNK, Jameson RM, et al (1954) Experiments pain referred from deep somatic tissues. J Bone Joint Surg 36-A:981–997 [PubMed]
- 7.Graven-Nielsen Brain Res. 1998;787:203. doi: 10.1016/s0006-8993(97)01480-7. [DOI] [PubMed] [Google Scholar]
- 8.Graven-Nielsen Brain Res. 1998;787:203. doi: 10.1016/s0006-8993(97)01480-7. [DOI] [PubMed] [Google Scholar]
- 9.Greene EC, (1963) Anatomy of the rat. Hafner, New York
- 10.Habler Neurosci Lett. 1988;94:119. doi: 10.1016/0304-3940(88)90281-9. [DOI] [PubMed] [Google Scholar]
- 11.Hökfelt Neuron. 1991;7:867. doi: 10.1016/0896-6273(91)90333-u. [DOI] [PubMed] [Google Scholar]
- 12.Jensen Peptides. 1990;11:1127. doi: 10.1016/0196-9781(90)90141-Q. [DOI] [PubMed] [Google Scholar]
- 13.Kellgren Clin Sci. 1938;3:175. [Google Scholar]
- 14.Kellgren Clin Sci. 1939;4:35. [Google Scholar]
- 15.Laursen Eur J Pain. 1997;1:105. doi: 10.1016/s1090-3801(97)90068-9. [DOI] [PubMed] [Google Scholar]
- 16.Lee Neurosci. 1985;15:1227. doi: 10.1016/0306-4522(85)90265-9. [DOI] [PubMed] [Google Scholar]
- 17.Lippitt Spine. 1984;9:746. doi: 10.1097/00007632-198410000-00016. [DOI] [PubMed] [Google Scholar]
- 18.McCarthy Neuroscience. 1990;34:623. doi: 10.1016/0306-4522(90)90169-5. [DOI] [PubMed] [Google Scholar]
- 19.Mooney V, Robertson J (1976) The facet syndrome. Clin Orthop Res 149–156 [PubMed]
- 20.Ohtori Auton Neurosci Basic Clin. 2000;86:13. doi: 10.1016/S1566-0702(00)00194-6. [DOI] [PubMed] [Google Scholar]
- 21.Ohtori Spine. 2001;26:946. doi: 10.1097/00007632-200104150-00020. [DOI] [PubMed] [Google Scholar]
- 22.OhtoriSpine 20002614711154533 [Google Scholar]
- 23.OhtoriAnn Anat 200018423512056753 [Google Scholar]
- 24.Pierau Neurosci Lett. 1982;31:123. doi: 10.1016/0304-3940(82)90103-3. [DOI] [PubMed] [Google Scholar]
- 25.Pierau Brain Res. 1984;321:63. doi: 10.1016/0006-8993(84)90681-4. [DOI] [PubMed] [Google Scholar]
- 26.Ruch TC (1946) Visceral sensation and referred pain. In: Fulton JF (ed) Howell’s textbook of physiology, 15th edn. Saunders, Philadelphia, pp 385–401
- 27.Rucker Brain Res. 1982;243:155. doi: 10.1016/0006-8993(82)91130-1. [DOI] [PubMed] [Google Scholar]
- 28.Sameda Spine. 2001;26:1105. doi: 10.1097/00007632-200105150-00003. [DOI] [PubMed] [Google Scholar]
- 29.Schmid Cell Tissue Res. 1983;232:9. doi: 10.1007/BF00222370. [DOI] [PubMed] [Google Scholar]
- 30.Selzer Brain Res. 1969;14:331. doi: 10.1016/0006-8993(69)90114-0. [DOI] [PubMed] [Google Scholar]
- 31.Simone J Neurophysiol. 1994;72:883. doi: 10.1152/jn.1994.72.2.883. [DOI] [PubMed] [Google Scholar]
- 32.Sinclair Brain. 1948;71:184. doi: 10.1093/brain/71.2.184. [DOI] [PubMed] [Google Scholar]
- 33.Suseki Spine. 1997;22:477. doi: 10.1097/00007632-199703010-00003. [DOI] [PubMed] [Google Scholar]
- 34.Taylor Neurosci Lett. 1982;33:1. doi: 10.1016/0304-3940(82)90120-3. [DOI] [PubMed] [Google Scholar]
- 35.Taylor J Neurosci Methods. 1983;8:211. doi: 10.1016/0165-0270(83)90035-3. [DOI] [PubMed] [Google Scholar]
- 36.Wiberg Acta Orthop Scand. 1949;19:211. doi: 10.3109/17453674908991094. [DOI] [PubMed] [Google Scholar]