Abstract
Eighty adult patients with lumbar disc herniation verified by magnetic resonance imaging (MRI) and clinical findings corresponding to the radiological level underwent microscopic disc removal to evaluate the outcome of perioperatively given corticosteroids in a prospective randomized double-blind study. In the treatment group the patient received 250 mg Solu-Medrol intravenously and 160 mg Depo-Medrol intramuscularly. Before closure of the wound, a free fat transplant soaked in 80 mg Depo-Medrol was placed on the dural sac. In the control group the same procedure was performed, but sodium chloride was given instead of Depo-Medrol. All patients underwent a clinical examination before surgery and at 2, 6, 12, 26, 52 and 104 weeks postoperatively, rating their pain with the visual analog scale (VAS) and function with the Disability Rating Index (DRI). The postoperative hospital stay was significantly shorter (P=0.01) in the treatment group (1.7 days) compared to the control group (2.3 days). Time taken to return to full-time work was also significantly shorter in the treatment group (P=0.003). VAS-W (Worst Pain during last week) was significantly lower in the treatment group (P=0.02). Postoperative spondylitis occurred in one patient in the control group and no adverse corticosteroids effect was seen. Our study shows that perioperatively given corticosteroids improve the outcome of microscopic disc surgery in terms of length of hospital stay and time taken to return to full-time work. The results also indicate that corticosteroid treatment reduces pain and improves functional outcome.
Keywords: Perioperative corticosteroids, Microscopic discectomy, Postoperative outcome, Lumbar disc herniation
Introduction
Besides careful patient selection and refined surgical techniques including free fat transplants in lumbar discectomy [1, 2, 13, 16, 17, 30, 34, 36], administration of steroids and/or injection of long-lasting local anesthetics have been additive treatment modalities for a long time [8, 9, 10, 18, 20, 22, 23, 25, 35]. Epidural and periradicular infiltration of steroids without surgery has also been a treatment modality [6, 15]. The rationale has been to reduce postoperative pain and minimize the early inflammatory reaction and late scar tissue formation, which might be causative factors in cases with persistent pain and functional disability. Several reports give biochemical evidence of inflammation at the site of lumbar disc herniations [11, 21, 27, 29, 31] and cytokines and growth factors may play a role in sciatic pain [3, 4, 28, 33]. The idea of steroid treatment is supported by Olmarker et al. [26, 27], who reported a morphological and neurophysiological study on pigs with nucleus pulposus applied on nerve roots, which caused a significant reduction of nerve conduction velocity that was prevented by early administration of steroids [26].
The result of peri- and/or postoperative steroid treatment as regards need for narcotic analgesics postoperatively and length of hospital stay have been favorable [10, 35], but the results of a few long-term studies have been discouraging [20, 25]. However, comparisons between earlier investigations are hampered by differences in diagnostic criteria of disc herniations, operative technique, and postoperative care. Furthermore, the administration of steroids varies, and the number of patients in each study is in most cases low [8, 9, 18, 23].
Our purpose was to examine whether corticosteroids administered perioperatively in patients undergoing microsurgical discectomy improves the outcome in terms of pain relief, function, hospital stay and sick-leave over a minimum 2-year follow-up.
Patients and methods
From October 1994 through November 1998, 85 patients with sciatica were invited to participate in a prospective randomized double-blind study at Örebro University Hospital. Approval was given by the Regional Ethics Committee of Örebro County Council. Inclusion criteria were: L3-4, L4-5 and L5-S1 lumbar disc herniation verified by magnetic resonance imaging (MRI), no previous surgery at the affected level, duration of sciatica of less than 1 year, clinical presentation of sciatica consistent with MRI findings and age between 18 and 65 years. All patients underwent a thorough clinical examination the day before surgery and again postoperatively after 2 weeks, 6 weeks, 3 months, 6 months, 12 months and 2 years.
The intensity of pain was assessed on a visual-analog scale ranging from 0 (no pain) to 100 (worst pain possible) documenting pain just now (VAS-N) and worst pain during the last week (VAS-W) [12, 24].
Impairment was assessed with the Disability Rating Index (DRI), which consists of 12 items concerning physical function in a self-administered form [24, 32]. Working status was either full-time work or sick-listed, completely or partially. Length of postoperative hospital stay in days was recorded. The degree of anxiety and/or depression was assessed with the Hospital Anxiety and Depression scale (HAD) [37].
The microsurgical discectomy was preceded by injection of 20 ml 0.5% bupivacaine (Marcaine) with 0.5% adrenaline into the subcutaneous tissue and paraspinal muscles of the incision site. Patients in the treatment group received 160 mg intramuscular methylprednisolone acetate (Depo-Medrol) and 250 mg intravenous methylprednisolone sodium succinate (Solu-Medrol). Following discectomy a free fat graft soaked in 80 mg methylprednisolone acetate (Depo-Medrol) was placed over the affected nerve root. The same procedure was performed in the patients of the control group, but methylprednisolone was substituted for saline solution similar to Glasser et al. [10]. To minimize infection, all patients received 1.5 g of cloxacillinatrium (Ekvacillin) intravenously three times on the day of surgery.
We used an operation microscope and all the examinations were performed by the same surgeon (A.L.), and blinding of the observer and the patients was maintained during the 2-year follow-up.
Statistical analysis
We expressed the outcomes VAS-N, VAS-W and DRI measured from 2 up to 104 weeks after surgery as change from baseline, and analyzed them by ANOVA for repeated measurement with group as main effect. The outcome "postoperative hospital stay" was measured in days from surgery and analyzed with the rank-based Mann-Whitney U-test. The outcome regarding time taken to return to full-time work was measured at discrete times. We used the life-table method and log rank test for this outcome measurement, because the calculations excluded patients who did not return to full-time work during the study period. In cases of missing data, calculations were made on the assumption that the lost data were missing at random.
Results
Eighty-five patients were initially included in the study, but five patients were excluded before randomization owing to suspicion of perioperative dural rifts. Hence the study finally comprised 38 patients receiving active treatment and 42 patients in the control group. The patient characteristics are shown in Table 1. There was no postoperative wound infection among patients receiving steroids, but one patient in the control group developed spondylitis 6 months postoperatively.
Table 1.
Characteristics and clinical findings of 80 patients with lumbar disc herniation: data for age, duration, pain score and Disability Rating Index represent mean values (VAS-N pain just now, VAS-W worst pain during the last week)
| Steroid group (n=38) | Control group (n=42) | |
|---|---|---|
| Age (years) | 40.1 | 42.1 |
| Male/female | 25/13 | 19/23 |
| Duration of sciatica (months) | 4.6 | 4.5 |
| VAS-N (0–100) | 53 | 49 |
| VAS-W (0–100) | 82 | 81 |
| Disability Rating Index (0–100) | 71 | 67 |
| Positive Lasègue's sign | 35 (92%) | 38 (90%) |
| Positive crossed Lasègue's sign | 10 (26%) | 10 (24%) |
| Sensory disturbance | 33 (87%) | 36 (86%) |
| Motor disturbance | 21 (55%) | 26 (62%) |
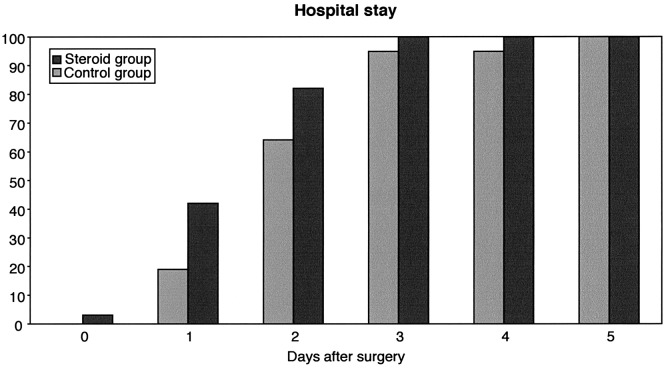
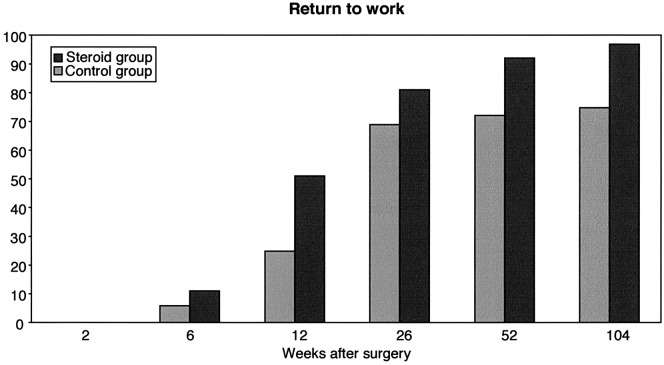
The mean postoperative hospital stay (Fig. 1) was 1.7 days for patients in the treatment group compared to 2.3 days for patients in the control group, which is statistically significant (P=0.01). Concerning the time taken to return to full-time work, we studied 73 patients out of 80; seven patients were excluded either because they were sick-listed for diagnoses other than the herniated disc, or they were students or were on a disability pension or because data were missing on their preoperative work status. In the study group, 36 out of 37 patients went back to full-time work compared to 30 out of 36 in the control group. Time taken to return to full-time work (Fig. 2) was significantly shorter in the study group (P=0.003).
Fig. 1.
Cumulative percentage of 80 patients discharged from hospital after microsurgical discectomy (steroid group n=38, control group n=42)
Fig. 2.
Cumulative percentage of 80 patients returned to full-time work after microsurgical discectomy (steroid group n=37, control group n=36)
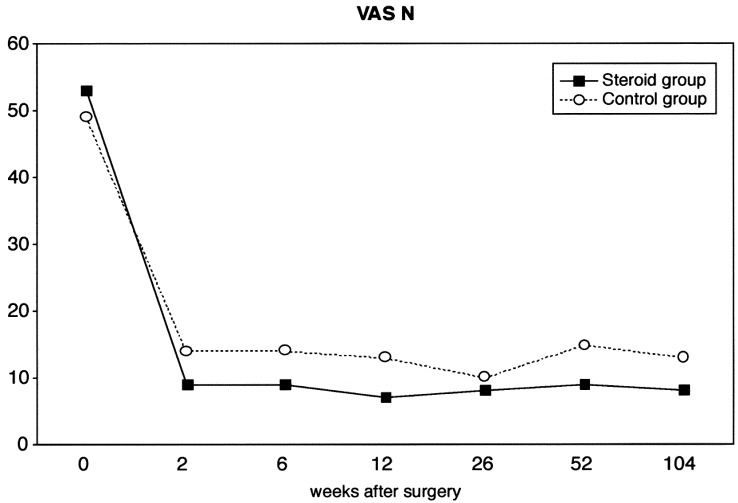
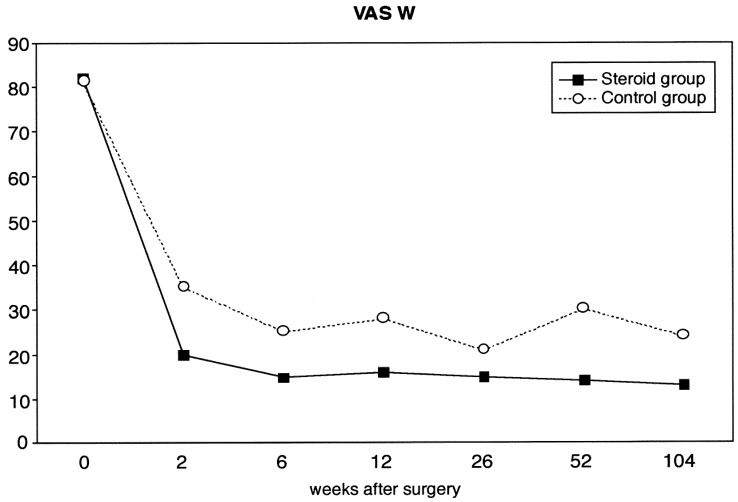
Pain just now (VAS-N) (Fig. 3), measured by the visual analog scale, was lower in the study group at all examinations, but this difference was not statistically significant (P=0.13). Worst pain during the last week (VAS-W) (Fig. 4) was statistically lower in the treatment group compared to the control group (P=0.02).
Fig. 3.
Mean values of current pain measured by visual analog scale (range 0–100) of 80 patients undergoing microsurgical discectomy (steroid group n=38, control group n=42)
Fig. 4.
Mean values of worst pain during the last week measured by visual analog scale (range 0–100) of 80 patients undergoing microsurgical discectomy (steroid group n=38, control group n=42)
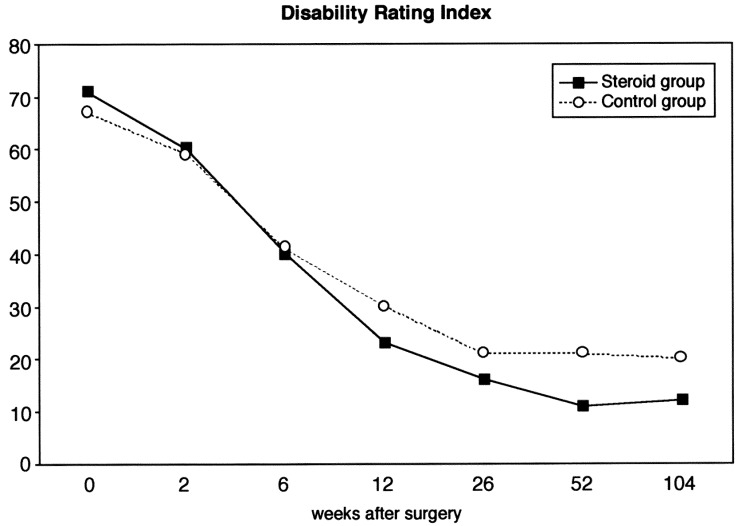
The Disability Rating Index (DRI) (Fig. 5) was equal in the two groups during the postoperative examinations at 2 weeks and 6 weeks. However, at all later examinations it was lower in the study group, but the difference was not statistically significant (P=0.08).
Fig. 5.
Mean values of Disability Rating Index (range 0–100) of 80 patients undergoing microsurgical discectomy (steroid group n=38, control group n=42)
Postoperative anxiety and depression scores were dichotomized to either no anxiety/depression ≤7 or borderline/case ≥8 [37]. In the study group there were 9 out of 38 patients (24%) who had one or more borderline/case anxiety scores during follow-up and 10 out of 42 patients (24%) in the control group. The results for borderline/case depression was six patients (16%) in the study group and seven patients (17%) in the control group. However, the first postoperative assessment was not performed until 2 weeks after surgery, because an early euphoria associated with steroid treatment may have been present during this initial postoperative period.
Discussion
Our main findings were that the patients in the steroid-treated group had a significantly shorter hospital stay and that they returned to work earlier than the patients in the control group. Furthermore, pain measured as the worst pain during the last week (VAS-W) was significantly lower in the treatment group.
These findings are in accordance with the report by Watters et al. [35], who studied the short-term effects of dexamethasone administered four times intravenously during the 1st postoperative day followed by four daily oral doses on a tapering schedule for another 2 days. The results also concur with those of Glasser et al. [10], who used perioperative parenteral corticosteroids and bupivacaine in microdiscectomy, thus reducing postoperative discomfort and length of hospital stay. However, Naylor et al. [25] found no significant effect of oral dexamethasone on a tapered scheme for 6 days postoperatively, nor did Manniche et al. [20] who, in a randomized study, administered prednisolone treatment for 4 weeks starting the day before surgery. There also seems to be a favorable lasting effect of a perioperative administration of long-term acting steroids without a need for postoperative oral medication, as patients given steroids returned to full-time work earlier than patients without active treatment.
As we administered steroids intravenously, intramuscularly and locally, the increased amount of steroids may have been beneficial both in inhibiting the biochemical irritation caused by nucleus pulposus and in decreasing the postoperative scar formation. With the aim of reducing postoperative epidural scar formation and protecting the spinal nerve, we also applied a fat transplant epidurally before closure of the wound [2, 13, 14, 16, 17]. Owing to our study design, we were not able to evaluate the quantity of locally delivered corticosteroid and we are unable assess whether the steroid-soaked fat transplant increased the main effect of the parenteral steroids or had any effect on scar formation.
The benefits of locally applied epidural methylprednisolone in lumbar discectomy are equivocal. Davis and Emmons [8], in a matched controlled discectomy study, showed that intraoperatively applied epidural methylprednisolone acetate (80 mg) led to a reduced need for pain and spasm medication and shorter hospital stay, but Lavyne and Bilski [18], who applied low-dose steroids locally, failed to improve the postoperative morbidity.
We did not observe any side effects of the steroid treatment, but a slight euphoria that might have promoted postoperative mobilization can of course not be excluded. There were, however, no differences between the steroid group and the control group in anxiety or depression scores. Other side effects like glucose intolerance and hypertension associated with short-term corticosteroid use are generally mild and completely reversible upon discontinuation. Avascular necrosis is a rare but severe and dose-dependent side effect seen in long-term corticosteroid use. As our patients received the steroid treatment on a single occasion, the risk of such side effects was very low [5].
The use of perioperative epidural steroid administration may predispose to infection [19]. We had no infection in the steroid group, but one case of spondylitis 6 months postoperatively in the control group. There were no diabetic patients in the study, and the amount of steroid carried by means of the soaked fat transplant is probably lower than what is obtained by instillation of epidural injections. Our use of prophylactic cloxacillinatrium probably also minimized the risk of perioperative infection.
There was a significant difference in favor of the steroid treated group as regards visual analogue pain scores for the previous week (VAS-W) during the 1-year follow-up, but not as regards pain scores just now (VAS-N). This finding probably explains the earlier return to work after discectomy of patients in the steroid group. VAS-W also represents the overall worst pain experienced during past week, which may be a more representative measurement than VAS-N. The diminished psychological impact of the microdiscectomy in the steroid-treated group was associated with less pain, and was beneficial both to shortening the hospital stay and to getting the patients back to work earlier than in the control group. The DRI showed a tendency in favor of the study group, but the difference was not statistically significant. However, the DRI estimates perceived disability, which may not be directly correlated with other outcome measures like length of hospital stay and time to return to full-time work.
In a study of nerve function in an experimental pig model, Olmarker et al. [26] found that high-dose methylprednisolone administered within 24 h dramatically improved nerve conduction velocity that had been significantly lowered by the application of nucleus pulposus on the cauda equina. They also observed that the scar tissue formation appeared less pronounced than in nontreated pigs. Cornefjord et al. [7] showed that diclofenac, a potent anti-inflammatory drug, reduced the nucleus pulposus-induced nerve root dysfunction.
We conclude that systemic perioperative treatment with corticosteroids at lumbar discectomy reduces pain, and shortens length of hospital stay and the time taken to return to work. Until more effective and potentially less harmful pharmacological agents are developed, systemic corticosteroids appear to be of benefit in disc surgery.
Acknowledgements
The authors thank Professor Steven J. Linton, PhD, Department of Occupational and Environmental Medicine, Örebro University Hospital, for valuable advice. The study was supported by Örebro County Council.
Footnotes
This paper was presented at the EuroSpine 2001 Annual Meeting, Gothenburg, Sweden
References
- 1.Andersson Spine. 1996;21:75S. doi: 10.1097/00007632-199612151-00009. [DOI] [PubMed] [Google Scholar]
- 2.Bernsmann Arch Orthop Trauma Surg. 2001;121:476. doi: 10.1007/s004020100277. [DOI] [PubMed] [Google Scholar]
- 3.Brisby H (2000) Nerve tissue injury markers, inflammatory mechanisms and immunologic factors in lumbar disc herniation. Thesis, Gothenburg University
- 4.Brisby Eur Spine J. 2002;11:62. doi: 10.1007/s005860100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchman J Clin Gastroenterol. 2001;33:289. doi: 10.1097/00004836-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Carette N Engl J Med. 1997;336:1630. doi: 10.1056/NEJM199706053362303. [DOI] [PubMed] [Google Scholar]
- 7.Cornefjord Eur Spine J. 2002;11:57. doi: 10.1007/s005860100299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis J Spinal Disord. 1990;3:299. [PubMed] [Google Scholar]
- 9.FoulkesClin Orthop 19892612242245548 [Google Scholar]
- 10.Glasser J Neurosurgery. 1993;78:383. doi: 10.3171/jns.1993.78.3.0383. [DOI] [PubMed] [Google Scholar]
- 11.Grönblad Spine. 1994;19:2744. doi: 10.1097/00007632-199412150-00002. [DOI] [PubMed] [Google Scholar]
- 12.Huskisson Lancet. 1974;7889:1127. doi: 10.1016/s0140-6736(74)90884-8. [DOI] [PubMed] [Google Scholar]
- 13.Jensen Spine. 1996;21:1072. doi: 10.1097/00007632-199605010-00016. [DOI] [PubMed] [Google Scholar]
- 14.Kanamori Spine. 2001;26:2258. doi: 10.1097/00007632-200110150-00018. [DOI] [PubMed] [Google Scholar]
- 15.Karppinen Spine. 2001;26:1059. doi: 10.1097/00007632-200105010-00015. [DOI] [PubMed] [Google Scholar]
- 16.Kiviluoto Acta Orthop Scand Suppl. 1976;164:66. doi: 10.3109/ort.1976.47.suppl-164.01. [DOI] [PubMed] [Google Scholar]
- 17.Langenskiöld Clin Orthop. 1976;115:92. [PubMed] [Google Scholar]
- 18.Lavyne J Neurosurgery. 1992;77:90. doi: 10.3171/jns.1992.77.1.0090. [DOI] [PubMed] [Google Scholar]
- 19.Lowell Spine. 2000;25:516. doi: 10.1097/00007632-200002150-00020. [DOI] [PubMed] [Google Scholar]
- 20.Manniche Scand J Rheumatol. 1994;23:30. doi: 10.3109/03009749409102132. [DOI] [PubMed] [Google Scholar]
- 21.McCarron Spine. 1987;12:760. doi: 10.1097/00007632-198710000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Milligan J Bone Joint Surg Br. 1993;75:769. doi: 10.1302/0301-620X.75B5.8376436. [DOI] [PubMed] [Google Scholar]
- 23.Mirzai Spine. 2002;27:343. doi: 10.1097/00007632-200202150-00003. [DOI] [PubMed] [Google Scholar]
- 24.Möller Spine. 2000;25:1711. doi: 10.1097/00007632-200007010-00016. [DOI] [PubMed] [Google Scholar]
- 25.NaylorOrthop Clin North Am 197783323768 [Google Scholar]
- 26.Olmarker Spine. 1994;19:1803. doi: 10.1097/00007632-199408150-00003. [DOI] [PubMed] [Google Scholar]
- 27.Olmarker Spine. 1995;20:665. doi: 10.1097/00007632-199503150-00006. [DOI] [PubMed] [Google Scholar]
- 28.Ozaktay Eur Spine J. 2002;11:467. doi: 10.1007/s00586-002-0430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piperno Spine. 1997;22:2061. doi: 10.1097/00007632-199709150-00001. [DOI] [PubMed] [Google Scholar]
- 30.Postacchini Spine. 1996;21:1383. doi: 10.1097/00007632-199606010-00023. [DOI] [PubMed] [Google Scholar]
- 31.SaalSpine 1990156742218714 [Google Scholar]
- 32.Salén J Clin Epidemiol. 1994;47:1423. doi: 10.1016/0895-4356(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 33.Specchia Eur Spine J. 2002;11:145. doi: 10.1007/s00586-001-0361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tullberg T, Isacson J, Weidenhielm L (1993) Does microscopic removal of lumbar disc herniation lead to better results than the standard procedure? Spine 18:24–27 [DOI] [PubMed]
- 35.Watters III WC, Temple AP, Granberry M (1989) The use of dexamethasone in primary lumbar disc surgery. Spine14:440–442 [DOI] [PubMed]
- 36.Weber Spine. 1983;8:131. [PubMed] [Google Scholar]
- 37.Zigmond Acta Psychiatr Scand. 1983;67:361. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]