Abstract
Posterior lumbar interbody fusion (PLIF) restores disc height, the load bearing ability of anterior ligaments and muscles, root canal dimensions, and spinal balance. It immobilizes the painful degenerate spinal segment and decompresses the nerve roots. Anterior lumbar interbody fusion (ALIF) does the same, but could have complications of graft extrusion, compression and instability contributing to pseudarthrosis in the absence of instrumentation. The purpose of this study was to assess and compare the outcome of instrumented circumferential fusion through a posterior approach [PLIF and posterolateral fusion (PLF)] with instrumented ALIF using the Hartshill horseshoe cage, for comparable degrees of internal disc disruption and clinical disability. It was designed as a prospective study, comparing the outcome of two methods of instrumented interbody fusion for internal disc disruption. Between April 1994 and June 1998, the senior author (N.R.B.) performed 39 instrumented ALIF procedures and 35 instrumented circumferential fusion with PLIF procedures. The second author, an independent assessor (S.M.), performed the entire review. Preoperative radiographic assessment included plain radiographs, magnetic resonance imaging (MRI) and provocative discography in all the patients. The outcome in the two groups was compared in terms of radiological improvement and clinical improvement, measured on the basis of improvement of back pain and work capacity. Preoperatively, patients were asked to fill out a questionnaire giving their demographic details, maximum walking distance and current employment status in order to establish the comparability of the two groups. Patient assessment was with the Oswestry Disability Index, quality of life questionnaire (subjective), pain drawing, visual analogue scale, disability benefit, compensation status, and psychological profile. The results of the study showed a satisfactory outcome (score≤30) on the subjective (quality of life questionnaire) score of 71.8% (28 patients) in the ALIF group and 74.3% (26 patients) in the PLIF group (P>0.05). On categorising Oswestry Index scores into "excellent", "better", "same", and "worse", we found no difference in outcome between the two groups: 79.5% (n=31) had satisfactory outcome with ALIF and 80% (n=28) had satisfactory outcome with PLIF. The rate of return to work was no different in the two groups. On radiological assessment, we found two nonunions in the circumferential fusion (PLIF) group (94.3% fusion rate) and indirect evidence of no nonunions in the ALIF group. There was no significant difference between the compensation rate and disability benefit rate between the two groups. There were three complications in ALIF group and four in the PLIF (circumferential) group. On the basis of these results, we conclude that it is possible to treat discogenic back pain by anterior interbody fusion with Hartshill horseshoe cage or with circumferential fusion using instrumented PLIF.
Keywords: Disc degeneration, Interbody fusion, Cages
Introduction
The management of chronic disabling low back pain has been problematic and controversial. This group of patients account for about 85–90% of the direct and indirect costs of managing low back pain [45, 46].
Crock [7, 8] described the condition of internal disc disruption. He postulated that abnormalities in the internal architecture of the disc could cause mechanical back pain and sclerotermal or referred buttocks and leg pain. However, this disc degeneration could occur due to aging [14]. Weinstein et al. [55] reported that the outer third of annulus of the vertebral disc has nociceptive capability, and this could account for the discogenic back pain due to internal disc disruption.
Kirkaldy-Willis and Hill [24] described three stages of disc degeneration. The first stage is dysfunction. It is characterised by circumferential and radial tears in the disc annulus and localised synovitis and hypermobility of the facet joints. The second stage is instability. There is internal disruption of the disc, progressive disc resorption, degeneration of the facet joints with capsular laxity, subluxation, and joint erosion. The final stage is stabilisation. This stage comprises osteophytosis and spinal stenosis.
Several authors have described successful treatment with instrumented anterior and posterior lumbar interbody fusions (PLIF) [9, 10, 11, 12, 13]. PLIF offers several advantages [1, 10, 16, 50, 52]. It restores disc height, the load bearing ability of anterior ligaments and muscles, root canal dimensions, and spinal balance. It immobilises the painful degenerate spinal segment, and decompresses the nerve roots. Early results of instrumented PLIF and use of a carbon-fibre cage for interbody fusion have been encouraging [1, 3, 52].
The success rates of anterior lumbar interbody fusion (ALIF) reported in the literature are quite variable. Penta and Fraser [42] reported a 68% patient satisfaction rate and 72.4% overall fusion rate at a minimum follow-up of 10 years. Tiusanen et al. [51] used posterior external fixators for stabilisation in ALIF. Solid fusion was obtained in 71% of patients, and 74% of patients were clinically very much improved at a minimum follow-up of 2 years (mean 5 years). Turner et al. [53] analysed 47 articles and found that, on average, 68% of patients had achieved a satisfactory outcome after lumbar fusion surgery (range 16–95%). Inoue and Watanabe [23] in 1982 reported on a large series of 350 patients who underwent anterior discectomy and interbody fusion for lumbar disc herniation. They achieved a 94.3% fusion rate, with the clinical results being good in 73% of patients.
Posterior fusion and instrumentation is a satisfactory method of treatment for low back pain. However, the outcome may be disappointing if the disc itself is the cause of pain. Flynn and Hoque [13], Stauffer and Coventry [47], and Newman and Grinstead [38] have reported complications of graft extrusion, compression and instability contributing to pseudarthrosis associated with ALIF without instrumentation. The Hartshill horseshoe cage was designed to overcome these problems [33]. It is a horseshoe-shape cage made of titanium that is inserted after removal of the disc in ALIF. Tricortical iliac crest graft is inserted in the confines of the implant. The cage is stabilised by inserting screws into lumbar vertebral bodies through holes in the implant. To standardise our results to those recently reported in the literature, we used validated objective scores and also assessed the disability benefit, compensation status and psychological distress of these patients.
The purpose of our study was to assess and compare the outcome of instrumented circumferential fusion through posterior approach (PLIF) with instrumented anterior lumbar interbody fusion using the Hartshill horseshoe cage for comparable degree of internal disc disruption and clinical disability.
Materials and methods
Between April 1994 and June 1998, the senior author (N.R.B.) performed 39 instrumented anterior lumbar interbody fusion (ALIF) procedures and 35 instrumented circumferential fusion with posterior lumbar interbody fusion (PLIF) procedures. The selection criteria for each procedure were identical. A protocol assigning patients to one procedure or another on the basis of strict randomisation, which might have been considered appropriate for comparing the two procedures, was not possible because of logistic problems with the availability of the implants and equipment. Also, patients were told about the two procedures and quite often they expressed their preference for one over the other.
In order to ensure the two groups were comparable, therefore, they were compared for age and sex distribution, number and level of segments fused, and grade of degeneration on magnetic resonance (MR) images (graded as 1–4 using a modification of the classification proposed by Paajanen [41] (Table 1).
Table 1.
Magnetic resonance imaging (MRI) classification of disc degeneration (modified from Paajanen et al. [41])
| MRI grade | Characteristics |
|---|---|
| 1 | Bright signal intensity, provocative discography positive |
| 2 | Grey signal intensity |
| 3 | Dark signal intensity + annular tears |
| 4 | Disc prolapse, loss of disc height, instability, and osteophytes |
An independent assessor (S.M.) performed the entire review. Preoperative radiographic assessment included plain radiography, MRI scans and provocative discography in all the patients. The MRI scans were read by the radiologist and also by the senior author (N.R.B.).
Patients were briefed about the discography procedure and their consent was obtained. They were then given prophylactic antibiotics and a local anaesthetic. Needles (18-G outer and 22-G and 25-G inner discography needles) were placed in the three lower mobile segments of the lumbar spine in each subject through a posterolateral approach. A water-soluble contrast (Omnipaque) was used for disc injections (Fig. 2). Additional levels also were tested in some patients. Fluoroscopic images were obtained to check that the needle was placed in central one-third of the disc in the caudal/rostral, anterior/posterior planes and in line with the spinous processes in the left/right plane. Anteroposterior views of vertebral endplates were also obtained with a modified Ferguson view.
Fig. 2.
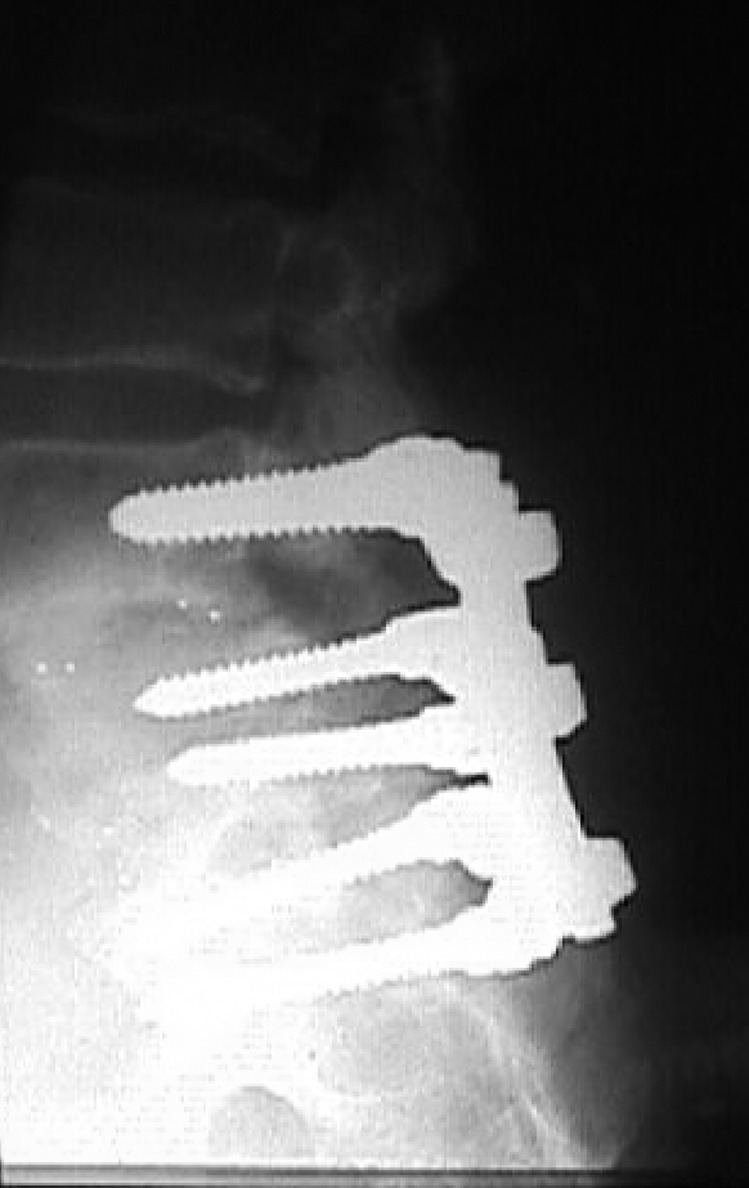
Posterior lumbar interbody fusion. This shows L4-L5 and L5-S1 circumferential fusion using posterior lumbar interbody fusion
The number of segments to be fused was decided by MRI, and provocative discography.
Patients
The inclusion criteria for the patients were:
Severe symptoms of low back pain not responding to medication, rehabilitation and conservative treatment
Low back pain present for at least 2 years
Minimum follow-up of 2 years
Age range of 24–67 years
Positive provocative discography and MRI scan correlating with patient's symptoms and signs
The exclusion criteria of patients were:
Disc herniation, spinal stenosis, and isthmic spondylolisthesis
Previous spinal operations like fusion, decompression or discectomy
We chose to include patients within the age range of 24–67 years, because we wanted to assess the outcome of these operations in people who are active in work.
There were 47 segment levels fused in the ALIF group and 52 segment levels in the PLIF group. Twenty-three segments in the ALIF group and 31 segment levels in the PLIF group had grade 4 degeneration, the rest of the levels had grade 3 degeneration.
Forty-two segment levels in the ALIF group and 45 levels in the PLIF group had typical concordant pain on provocative discography. Five segment levels in the ALIF group and seven levels in the PLIF group had atypical pain on discography. However, all these patients had grade 4 degeneration at the adjacent disc level and therefore, in order to prevent post-fusion symptoms at these levels, the arthrodesis was extended to involve those segment levels.
Surgical technique
ALIF with Hartshill horseshoe
The operation is performed through a direct anterior transperitoneal approach (Pfannenstiel) for L5-S1 and a standard anterolateral retroperitoneal approach for the other lumbar levels. Steinmann pins are inserted in the vertebral body and intra-operative radiographs are taken to confirm the level. The annulus is exposed and excised along with the nucleus pulposus right down to the posterior longitudinal ligament. The upper and lower endplates are cleared of all the cartilage up to the bleeding cancellous bone. A peripheral cortical rim is retained over the upper and lower surface of the adjacent vertebrae to seat the horseshoe cage. Tricortical iliac crest bone autograft was used for fusion along with small pieces of cancellous graft packed firmly in the horseshoe (Fig. 1).
Fig. 1.
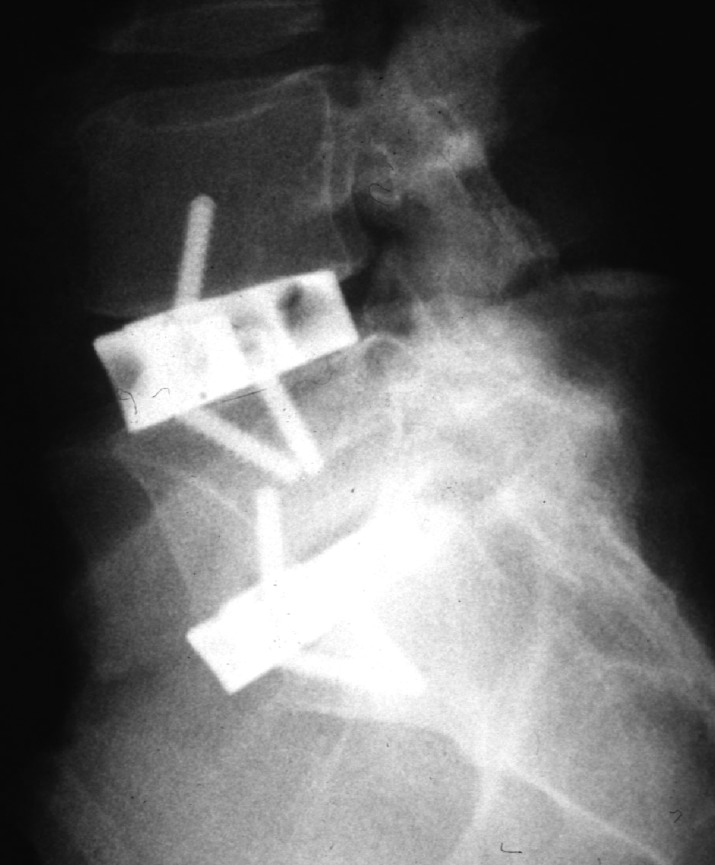
Hartshill horseshoe cage for anterior lumbar interbody fusion. This shows L4-L5 and L5-S1 fusion
PLIF with circumferential fusion
In the circumferential fusion group with PLIF, the approach was midline posterior. Laminectomy and a facetectomy were done. Intra-operative radiographs were taken to confirm the level. Isola rods and pedicle screws were used for stabilisation. In addition to this, before preparation of the bed for bone grafting, nerve roots were retracted and an incision was made over the disc. The entire disc was removed and end plates were curetted to the bleeding cancellous bone. Autologous iliac crest cancellous bone was inserted in carbon-fibre ramps, which were then placed in the disc spaces and between the transverse processes and the facet joints (Fig. 2). All patients in both groups were immobilised in a Boston thoracic lumbar sacral orthosis (TLSO) for 12 weeks.
Evaluation and comparison
The two groups were compared according to their radiological results and on the clinical outcome measures of improvement of back pain and work capacity.
Clinical evaluation
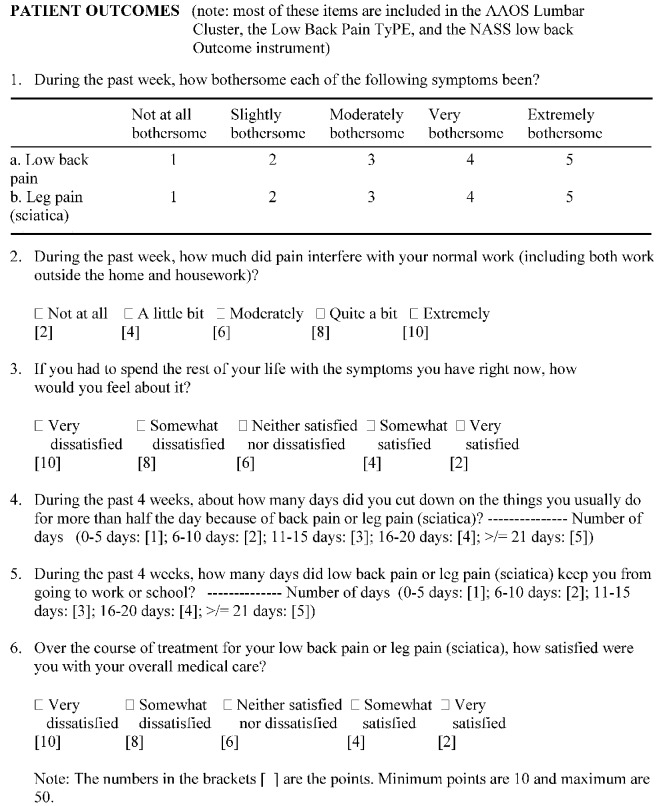
The patients were asked to fill out a questionnaire including demographic details (age, sex, smoking habits), walking distance and questions regarding current employment, and were asked to give their opinion regarding the outcome of the surgery using a "core set" of six questions as proposed by Deyo et al. [9]. This questionnaire covered the domains of pain symptoms, back-related function, generic well-being, disability (social role), and satisfaction with care (Fig. 3). The minimum score was 10 and the maximum 50. A postoperative score of ≤30 was considered to show a good overall improvement. This score also measured the patients' subjective improvement, and is shown as subjective score in Table 2.
Fig. 3.
The "core set" of outcome measures for low back pain, devised by Deyo et al. [9], were used to evaluate subjective patient outcome (note: most of these items are included in the AAOS Lumbar Cluster, the Low Back Pain TyPE, and the NASS low back outcome instrument)
Table 2.
Subjective and objective outcome parameters: mean values at follow-up (with ranges in parentheses) for the anterior lumbar interbody fusion with Hartshill horseshoe (ALIF) and the posterior lumbar interbody fusion (PLIF) groups. There were no significant differences between the two groups on any of the parameters (P<0.05) (MSPQ Modified Somatic Perception Questionnaire, ZDS Zung Depression Scale)
| Parameter | ALIF | PLIF |
|---|---|---|
| Walking distance | 1305 yds (1193 m) | 1228.6 yds (1123 m) |
| Oswestry Disability Index | 32.9 (0–82) | 30.5 (0–86) |
| Subjective score | 23.7 (8–50) | 23 (10–48) |
| Distress (MSPQ+ZDS)* | 28.5 (4–99) | 25.1 (0–81) |
| Visual analogue scale | 4.2 (0–10) | 4 (0–10) |
| Pain drawing | 5.2 (0–36) | 5.1 (0–19) |
Patients were also asked to complete a ten-point visual analogue pain scale, with 0 indicating "no pain at all" and 10 indicating "maximum pain possible". A pain drawing was used for them to show the site of pain. They were asked to describe the characteristics of the pain, which was scored as: numbness (4), pin prick (3), burning (2), and/or stabbing (1).
Patients were subjectively scored on the following parameters after the operation: excellent (3), better (2), same (1), worse after the operation (0). Objective assessment was made by using the Oswestry Disability Questionnaire [12]. A psychometric measure incorporating the Modified Somatic Perception Questionnaire (MSPQ) [34] and the Zung Depression Scale (ZDS) [57] was used to assess psychological distress at review. A combined score with a potential range of 0–99 is calculated, and distress is defined as a score of 29 or more for men and 33 or more for women. Preoperative and postoperative scores were available for all these patients. Sickness and disability benefit was also recorded for each patient preoperatively and at last follow-up.
Radiologic evaluation
Union was defined as solid when there was bony trabecular continuity and less than 4° mobility between the segments on flexion-extension stress radiographs [48]. The union was defined as probable when the bony trabecular continuity was not very clear but there was less than 4° mobility between the adjacent fused segments. Nonunion was defined as a visible gap, graft collapse, and motion greater than 4° [48].
Statistical analysis
The differences observed between the groups were analysed using the chi-square test, two by two Fisher's exact test and the Mann-Whitney U test.
Follow-up
The minimum follow-up in both groups was 2.0 years. The mean follow-up for the PLIF group was 2.4 years (range 2.0–3.1 years) and for the ALIF group it was 3.1 years (range 2.1–5.8 years).
Results
The results of the preoperative assessments showed that 14 patients in the ALIF group and 12 patients in PLIF group had parasthesia, and non-dermatomal sensory deficits in their lower limbs. There was no radiculopathy. The power, tone and reflexes were normal in all the patients. There were no differences between the two groups in their demographic characteristics, grade of MRI disc degeneration or number and level of segments fused (P>0.05) (Table 3).
Table 3.
Demographic characteristics, duration of preoperative back/leg pain, and number and level of segments fused for the two groups. There was no significant difference between the two groups on any of the parameters
| Parameter | ALIF | PLIF |
|---|---|---|
| Age in years: mean (range) | 43 (25–67) | 40.6 (24–67) |
| Females (n) | 23 | 12 |
| Males (n) | 16 | 23 |
| Years of preop pain: mean (range) | ||
| Back pain | 10.1 (2.1–32) | 5.8 (0.3–20) |
| Leg pain | 8.1 (0–32) | 4.2 (0–10.4) |
| Level(s) fused | ||
| L5–S1 | 22 | 35 |
| L4–L5 | 21 | 16 |
| L3–4 | 2 | 1 |
| L2–L3 | 2 | 0 |
| Single level | 31 | 18 |
| Two level | 8 | 17 |
Pain and function
Postoperatively, the mean back and leg pain measured by visual analogue scale (0–10) was 4.2 in the ALIF group and 4 in the PLIF group. At last follow-up, three patients had back pain and two had leg pain in the ALIF group. Six patients said their symptoms were the same after operation, and two patients said their symptoms were worse after the operation. In the PLIF group, three patients had back pain and two had leg pain at last follow-up. Four patients said that their symptoms had improved and two said that their symptoms were worse after the operation.
There was no significant difference in the mean Oswestry score between ALIF and PLIF groups of patients (P>0.05). The psychological profile and walking distance of both groups of patients were not dissimilar (no significant difference; P>0.05). The mean postoperative subjective score (quality of life assessment) was 27.4 in the ALIF group and 23 in the PLIF group. There were no statistical differences between pain improvement as measured with visual analogue scale, pain drawing, Oswestry score and subjective score between the two groups. There were no differences in the functional and psychological improvement between the two groups (Table 2).
Twenty-seven patients in the ALIF group were on disability benefit, of whom six did not improve and two were worse at last follow-up (29.6% unsatisfactory outcome). There was a significant difference in outcome between those on and those not on disability benefit. Twenty-two patients in the PLIF group were on disability benefit, of whom four did not show any improvement, and two were worse after operation (27.2% unsatisfactory outcome), however the difference in outcome between those on and those not on benefits was not significant in this group (Table 4). There were 12 patients on workers' compensation in the ALIF group, of whom one was worse and five did not show any improvement after operation (50% unsatisfactory outcome). Nine patients in the PLIF group were on workers' compensation, one of whom was worse and four of whom did not improve after the operation (55.5% unsatisfactory outcome). Patients on compensation had a significantly worse outcome than patients who were not on compensation in both groups (Table 5).
Table 4.
Outcome in the two groups according to disability benefit status. There was a significant difference in outcome between those on benefits and those not on benefits in the ALIF group (P<0.05), but no such difference was found in the PLIF group
| Outcome (Oswestry score)a | Benefits n (%) | No benefits n (%) | All patients n (%) |
|---|---|---|---|
| ALIF (P=0.0417 by Fisher's exact test) | |||
| Satisfied | 19 (70.3) | 12 (100) | 31 (79.5) |
| Unsatisfied | 8 (29.7) | 0 (0) | 8 (20.5) |
| PLIF (P=0.2197 by Fisher's exact test) | |||
| Satisfied | 16 (72.7) | 12 (92.3) | 28 (80) |
| Unsatisfied | 6 (27.3) | 1(7.7) | 7 (20) |
a Satisfied = excellent or better; unsatisfied = same or worse
Table 5.
Outcome in the two groups according to compensation status. Patients on compensation had a significantly worse outcome than patients who were not on compensation in both groups
| Outcome (Oswestry score) | Compensation n (%) | Non-compensation n (%) | All patients n (%) |
|---|---|---|---|
| ALIF (P=0.0056) by Fisher's exact test | |||
| Satisfied | 6 (50) | 25 (92.6) | 31 (79.5) |
| Unsatisfied | 6 (50) | 2 (7.4) | 8 (20.5) |
| PLIF (P=0.0064) by Fisher's exact test | |||
| Satisfied | 4 (45) | 24 (92.3) | 28 (80) |
| Unsatisfied | 5 (55.5) | 2 (7.7) | 7 (20) |
Fusion
Fusion was assessed by plain and stress radiographs. Computed tomography (CT) scan was done in patients with persistent symptoms and inconclusive radiographs. Patients in the ALIF group who had an unsatisfactory clinical outcome were investigated with bone scan and CT scan for pseudarthrosis, in addition to the above assessment, as radiological trabecular continuity is difficult to see with the titanium implant. There was no evidence of nonunion on these investigations.
One segment each (L4-L5 and L5-S1) in two patients in the PLIF group had doubtful interbody fusion, because of lack of trabecular continuity, but they both had solid posterior fusion. Both these patients reported significant improvement from their operation. Technically the PLIF had gone into nonunion, but the posterolateral fusion mass was intact and fused, thus stabilising that segment.
Clinical outcome
On subjective score assessment, there was a satisfactory outcome (score≤30) in 71.8% of patients(n=28) in the ALIF group and in 74.3% of patients (n=26) in the PLIF group (P>0.05). On assessment classifying the Oswestry index into the four categories shown in Table 6, we found no difference in outcome between the two groups: 79.5% of ALIF patients (n=31) had a satisfactory outcome ("excellent" or "better") and 80% of PLIF patients (n=28) had a satisfactory outcome.
Table 6.
Outcome gradings in the two groups based on the patients' Oswestry scores. There was no significant difference between the outcome grades of the two groups (P=0.1046)
| Improvement | ALIF | PLIF |
|---|---|---|
| Excellent | 16 | 23 |
| Better | 15 | 5 |
| Same | 6 | 5 |
| Worse | 2 | 2 |
Eleven patients (28.2%) went back to their original work and 14 patients (35.9%) went back to lighter work after the ALIF operation. Fourteen patients (40%) went back to their original work and ten patients (28.6%) went back to lighter work or changed to less strenuous jobs after the PLIF operation. Thus, there was a 64.1% rate of improvement in working ability in the ALIF group and 68.6% improvement in the PLIF group (P>0.05).
Complications
There was one case of postoperative pneumonia and one superficial infection in the ALIF group, which settled with antibiotics. One patient in the ALIF group had severe sciatica due to impingement from the screw used to stabilise the Hartshill cage. She was re-operated 3 weeks later and the screw was placed in the vertebral body, thus relieving her symptoms. There was one superficial infection in the PLIF group, which was treated successfully with antibiotics. Two patients in the PLIF group had urinary tract infections that were successfully treated with antibiotics. One patient in the PLIF group had persistent iliac crest donor site pain for 4 months.
Discussion
The advantage of circumferential fusion with instrumented PLIF is that through one posterior approach one can have interbody and posterolateral fusion. Use of a carbon-fibre cage secures and stabilises the graft and prevents loss of disc height until the bone graft fuses. We used pedicle screws and rods to stabilise the spine. Instrumented posterolateral fusion and PLIF can cause severe damage to spine musculature by denervation, which inevitably occurs during decortication of the transverse processes, and this could decondition the back [35, 40]. Another disadvantage could be that the extensive dissection and bone graft required for circumferential fusion could result in increased morbidity, although this did not happen in our group of patients.
The advantage of ALIF using the Hartshill horseshoe is that one stabilises and rigidly fixes the motion segment, and secures the bone graft, without any further procedure for stabilisation. The disadvantage is that one cannot do a circumferential fusion using the anterior approach, although it may not be needed with the use of the cage. One cannot conclusively identify the status of bony fusion with a titanium horseshoe cage, and thus one relies on indirect evidence of the implant being intact and not loose. CT scan would be expensive and time consuming to perform routinely to investigate the graft status.
The purpose of this study was to assess and compare the outcome of instrumented circumferential fusion through a posterior approach [PLIF and posterolateral fusion (PLF)] with instrumented ALIF using the Hartshill horseshoe cage, for comparable degrees of internal disc disruption and clinical disability.
The results showed a fusion rate of PLIF in circumferential fusion of 94.3% (33 patients). The fusion rate in the ALIF group cannot be conclusively proven. Anterior interbody fusion rates vary between 19% and 96%, depending on the method of evaluation, fixation technique, and graft material [4, 13, 15, 18, 20, 22, 26, 28, 30, 33, 38, 42, 47, 51]. However, radiological trabecular continuity is difficult to see with the titanium implant, making it difficult to assess how many patients with a satisfactory clinical outcome do not have a bony fusion. Biomechanically, an implant would be expected to fail if there is nonunion, and we did not have any failure or radiolucency around the screws or the cage at a minimum follow-up of 2 years. This leads us to assume that the motion segment is rigidly fixed and this can only happen if there is solid union at that level. Therefore, we assume that the fusion rate in this group was high. Tiusanen et al. [51] found that when they used three tricortical bone grafts for ALIF procedures they had 100% fusion. Corticocancellous bonegrafts were used in many of these reported techniques, to achieve anterior fusion [25, 30, 39]. Postoperative problems like height loss, graft collapse and pseudarthrosis led to the development of carbon-fibre cages [1, 52], and titanium rings [26, 33] filled with cancellous grafts. The Hartshill horseshoe cage in this study achieves immediate stabilisation, and the screws in the superior and inferior vertebral bodies provide rigid fixation. This instrumentation avoids the need for posterior stabilisation, which is often required with other cages or bone grafts in ALIF procedures [11, 21, 30]. The cage also prevents the graft sinkage through the vertebral endplates, prevents graft extrusion, opens up the neural foramina and recreates the normal lordosis of the lumbar segment.
Procedures that involve disc excision and instrumented interbody fusion (PLIF and ALIF) eliminate the chemical and mechanical source of pain associated with internal disc disruption. Cessation of abnormal motion of annular torn disc and removal of biochemical substances within the degenerated disc [36, 55, 56] should eliminate the nociceptive stimulation from the outer annulus. The pathogenesis of disc degeneration could arise from a major injury or small repeated injuries to the motion segment, which will produce tears in the annulus. The outer half of the annulus has a rich sensory innervation [43], which could cause low back pain [27, 36, 55]. Because disc is avascular, it will heal with inferior scar tissue, which may not tolerate the loads on the spine and thus could cause recurrent attacks of back pain. Since the motion segment is a three-joint complex comprising disc and two facet joints, the highest rate of fusion is obtained from supplementary fixation of the facet joints behind the anterior graft [37]. Supplementing this with posterolateral bone grafts will further improve the fusion rate. Our study included two patients who had doubtful interbody fusion but solid posterolateral arthrodeses. Leufven and Nordwall [29] reported a 93% fusion rate and 73% satisfactory outcome (combining excellent, good and fair results), and 62% return to work rate. Chow et al. [5] reported on 97 patients with degenerate lumbar intervertebral discs treated with anterior lumbar interbody fusion, who had a single-level fusion rate of 85% (75 patients) and relief of back pain in 89%. Fujimaki et al. [15] described 84 patients who underwent ALIF. They achieved a 95% fusion rate and a 95% clinical success rate determined by return to work status. Lin et al. [31] reported 89% excellent or good results in 46 patients who underwent PLIF. Collis [6] reported a 96% fusion rate and a 100% satisfactory clinical result in 25 patients who underwent PLIF. Loguidice et al. [32] and Selby et al. [44] reported a 74% satisfactory result with ALIF. Schechter et al. [43] reported 96% fusion and 92% satisfactory clinical result. Vamvanij et al. [54] reported an 88% fusion and a 63% success rate with the Bagby and Kuslich (BAK) cage and posterior facet fusion. Kuslich et al. [28] reported a 91% fusion rate, 84% pain relief and 91% functional improvement at 24 months' follow-up using the BAK method of lumbar interbody fusion. Other authors [1, 2, 3, 10, 16, 50, 52] have reported more than 70% satisfactory clinical results with instrumented interbody fusion. Our patients had a 72% rate of clinical satisfaction with ALIF and 74% with circumferential fusion (PLIF) using subjective scores, and 79.5% and 80% clinical satisfaction using the Oswestry Disability Index. Our results were somewhere in the middle of the spectrum of results reported in the literature.
There were two factors that could have affected the result. Firstly, compensation status is known to affect clinical outcome [17, 19]. In our study it was clearly shown that non-compensation patients had a significantly better clinical satisfaction rate, of over 92% (Table 5). Secondly, Tandon et al. [49] have shown that patients with chronic back pain become dependent on welfare benefits, and due to this often do not want to return to work. More than half of our patients were on disability benefit. There was a 70.3% satisfactory outcome measured by the Oswestry Disability score in the ALIF group and a 72.7% satisfactory outcome in the circumferential fusion (PLIF) group among the patients who were on disability benefit. When compared with the patients not on disability benefits we found that patients in the ALIF group had a significantly (P=0.0417) better outcome (100% satisfactory) if not on disability benefit, but the same did not apply to the PLIF group (92.3% satisfactory among those not on disability; (P=0.2197) (Table 4). Thus socioeconomic and psychosocial factors may have significantly affected our clinical result. However, as there was no significant difference between the compensation rate and disability benefit rate between the two groups, any effect these factors may have had will have operated in a similar manner in both groups, minimising their effect on how the outcomes of the two groups compared with one another.
We express caution in the interpretation of our results, as the patients were not randomised. However, the two groups were shown to be similar for demographic characteristics, disease severity, and number of levels fused.
The results showed no difference between them in the subjective outcome (core set of six questions: Table 2) and Oswestry disability index. The two groups were similar for complication rate: three in the ALIF group and four in the PLIF (circumferential) group. The rate of return to work assessment was similar in the two groups. The fusion rate was 94% in the PLIF group and possibly 100% in the ALIF group.
We therefore feel justified in concluding, on the basis of the results of this study, that anterior interbody fusion with the Hartshill horseshoe cage and circumferential fusion using instrumented PLIF are both acceptable in the treatment of discogenic back pain.
References
- 1.Brantigan Spine. 1993;18:2106. doi: 10.1097/00007632-199310001-00030. [DOI] [PubMed] [Google Scholar]
- 2.Brantigan JW, Steffee AD, Geiger JM (1991) Carbon fiber implant to aid interbody lumbar fusion: mechanical testing. Spine 16 [Suppl]:277–282 [DOI] [PubMed]
- 3.Brantigan JW, Steffee AD, Lewis ML, Quinn LM, Persenaire JM (1996) Lumbar interbody fusion using the Brantigan I/F cage for PLIF and the VSP pedicle screw system: two-year results of a food and drug administration IDE clinical trial. Prepublication data submitted to FDA
- 4.Calandruccio Anterior lumbar fusion Clin Orthop. 1964;35:63. [PubMed] [Google Scholar]
- 5.Chow Spine. 1980;5:452. doi: 10.1097/00007632-198009000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Collis Clin Orthop. 1985;193:64. [PubMed] [Google Scholar]
- 7.Crock Med J. 1970;1:983. [Google Scholar]
- 8.Crock J Bone Joint Surg Br. 1976;58:193. doi: 10.1302/0301-620X.58B2.932081. [DOI] [PubMed] [Google Scholar]
- 9.Deyo Spine. 1998;23:2003. doi: 10.1097/00007632-199809150-00018. [DOI] [PubMed] [Google Scholar]
- 10.Enker Clin Orthop. 1994;300:90. [PubMed] [Google Scholar]
- 11.Evans H (1989) Biomechanics of lumbar fusion. In: Lin PM, Gill K (eds) Lumbar interbody fusion. Principles and techniques in spine surgery. Aspen Rockville, pp 9–13
- 12.Fairbank Physiotherapy. 1980;66:271. [PubMed] [Google Scholar]
- 13.Flynn J Bone Joint Surg Am. 1979;61:1143. [PubMed] [Google Scholar]
- 14.Frymoyer Spine. 1981;6:284. [Google Scholar]
- 15.Fujimaki Clin Orthop. 1982;165:164. [PubMed] [Google Scholar]
- 16.Gertzbein Spine. 1996;21:1918. doi: 10.1097/00007632-199608150-00018. [DOI] [PubMed] [Google Scholar]
- 17.Greenough Spine. 1998;23:479. doi: 10.1097/00007632-199802150-00015. [DOI] [PubMed] [Google Scholar]
- 18.Hahnel Z Orthop Ihre Grenzgeb. 1991;129:197. doi: 10.1055/s-2008-1040183. [DOI] [PubMed] [Google Scholar]
- 19.HanleySpine 198914482521535 [Google Scholar]
- 20.Harmon Clin Orthop. 1963;26:107. [PubMed] [Google Scholar]
- 21.Holte Eur Spine J. 1994;3:32. doi: 10.1007/BF02428314. [DOI] [PubMed] [Google Scholar]
- 22.Hoover J Bone Joint Surg Am. 1968;50:194. [Google Scholar]
- 23.InoueClin Orthop 1984183226697590 [Google Scholar]
- 24.Kirkaldy-Willis Spine. 1979;4:102. doi: 10.1097/00007632-197903000-00003. [DOI] [PubMed] [Google Scholar]
- 25.KozakClin Orthop 1994300458131355 [Google Scholar]
- 26.Krause P, Halm H (1993) Combined anterior and posterior fusion with MPDS and titanium cage on lumbosacral instabilities. First International Meeting on Munster posterior and anterior double rod system. Munster
- 27.Kuslich Orthop Clin North Am. 1991;22:181. [PubMed] [Google Scholar]
- 28.Kuslich Spine. 1998;23:1267. doi: 10.1097/00007632-199806010-00019. [DOI] [PubMed] [Google Scholar]
- 29.Leufven Spine. 1999;24:2042. doi: 10.1097/00007632-199910010-00014. [DOI] [PubMed] [Google Scholar]
- 30.Liljenqvist Eur Spine J. 1998;7:125. doi: 10.1007/s005860050042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LinClin Orthop 19831801546354543 [Google Scholar]
- 32.Loguidice Spine. 1988;13:366. doi: 10.1097/00007632-198803000-00027. [DOI] [PubMed] [Google Scholar]
- 33.Madan J Spinal Disord. 2001;14:104. doi: 10.1097/00002517-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Main J Psychosom Res. 1983;27:503. doi: 10.1016/0022-3999(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 35.Mayer Spine. 1989;9:986. doi: 10.1097/00007632-198909000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Mooney Spine. 1987;12:54. [Google Scholar]
- 37.Nachemson Spine. 1996;21:1835. doi: 10.1097/00007632-199608010-00023. [DOI] [PubMed] [Google Scholar]
- 38.NewmanSpine 1992178311502649 [Google Scholar]
- 39.O'BrienEur Spine J 19921220054939 [Google Scholar]
- 40.Oliver CW, Greenough CG (1994) The role of lumbar paraspinal surface electromyography in low back pain. J Bone Joint Surg Br 76[Suppl]:44
- 41.Paajanen Spine. 1989;14:982. doi: 10.1097/00007632-198909000-00012. [DOI] [PubMed] [Google Scholar]
- 42.Penta Spine. 1997;22:2429. doi: 10.1097/00007632-199710150-00021. [DOI] [PubMed] [Google Scholar]
- 43.Schechter Orthopedics. 1991;14:447. doi: 10.3928/0147-7447-19910401-08. [DOI] [PubMed] [Google Scholar]
- 44.Selby DK, Henderson MJ, Blumenthal S, Dossett D (1988) Anterior lumbar fusion. In: Cauthen JC (ed) Lumbar spine surgery, 2nd edn. Williams and Wilkins, Baltimore, pp 248–260
- 45.Snook SH (1982) Low back pain in industry. In: White AA III, Gordon SL (eds) American Academy of Orthopaedic Surgeons Symposium on Idiopathic Low Back Pain. Mosby, St. Louis, pp 23–38
- 46.Spengler Spine. 1986;11:241. doi: 10.1097/00007632-198604000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Stauffer J Bone Joint Surg Am. 1972;54:756. [PubMed] [Google Scholar]
- 48.Suk Spine. 1997;22:210. doi: 10.1097/00007632-199701150-00016. [DOI] [PubMed] [Google Scholar]
- 49.Tandon Spine. 1999;24:1833. doi: 10.1097/00007632-199909010-00013. [DOI] [PubMed] [Google Scholar]
- 50.Temple Spine. 1994;19:537. doi: 10.1097/00007632-199403000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Tiusanen Clin Orthop. 1996;324:153. doi: 10.1097/00003086-199603000-00018. [DOI] [PubMed] [Google Scholar]
- 52.Tullberg Eur Spine J. 1996;5:178. doi: 10.1007/BF00395510. [DOI] [PubMed] [Google Scholar]
- 53.Turner JAMA. 1992;268:907. [PubMed] [Google Scholar]
- 54.Vamvanij J Spinal Disord. 1998;11:375. [PubMed] [Google Scholar]
- 55.Weinstein Spine. 1988;13:1344. doi: 10.1097/00007632-198812000-00002. [DOI] [PubMed] [Google Scholar]
- 56.Yoshizawa J Pathol. 1980;132:95. doi: 10.1002/path.1711320202. [DOI] [PubMed] [Google Scholar]
- 57.Zung Arch Gen Psychiatry. 1965;12:63. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]