Abstract
Pinealectomy frequently produces spinal deformity in some animal models, but the precise biological mechanism of this phenomenon remains obscure. The current study investigated the effects of an autograft pineal body on the development of spinal deformity and serum melatonin (MLT) concentration after pinealectomy in the chicken. Thirty-six chickens (2 days of age) were divided into three equal groups. While the removal of the pineal gland was performed in groups B and C, a pineal body autograft was surgically implanted into the body wall musculature only in the pineal transplantation group (group C). Chickens in which no surgical intervention was performed served as intact controls (group A). Posteroanterior radiographs of the spines of the chickens were taken at the age of 8 weeks. These were used to determine Cobb angles and to measure the rib-vertebra angles (RVA) on the concave and convex sides of the curves, from which data the difference between the convex and concave RVA (the RVAD) was calculated. At the end of the study, serum MLT levels were determined using the enzyme-linked immunosorbent assay method, and histopathological examination of specimens from all the groups was performed. The results were compared using one-way analysis of variance followed by Duncan's test for pairwise comparisons or by the Kruskal-Wallis test followed by the Mann-Whitney U tests for comparisons between two groups. In this study, the serum MLT levels in groups B and C were significantly lower than those in group A (P<0.05). However, scoliosis developed in only 7 of 12 (58%) in group B and 6 of 12 (50%) in group C. The average Cobb angle and RVAD in groups B and C were significantly larger than those found in group A (P=0.000 and P=0.001, respectively). Interestingly, there were no significant differences in either serum MLT levels or development of scoliosis between groups B and C. From the results of the current study, it is evident that the intramuscular pineal gland transplantation following pinealectomy in young Hybro Broiler chickens has no significant effect on the development of spinal deformity and serum MLT level. In the light of this result, the role of MLT in the development of spinal deformity in chickens after pinealectomy remains controversial, and further investigations are warranted.
Keywords: Chickens, Spinal diseases, Pineal body, Pinealectomy, Transplantation, Melatonin, Radiography
Introduction
In the human, the pineal gland, which is located at "the geometrical mid-point of intracranial cavity" [27], functions as a biological watch that regulates the secretion of many hormones [4, 6, 8, 35]. Over three centuries ago, the French philosopher Descartes described the pineal gland as "the seat of the soul" [4]. Melatonin (MLT) has a unique position among the hormones of the diffuse neuroendocrine system. It displays a circadian pattern of secretion, with low levels during the day and high concentrations at night [1, 2, 3, 4, 5, 6, 8, 18, 22, 35]. Furthermore, there is an age-dependent alteration of its circadian secretion during the human lifetime. Since the first experiment by Thillard [30] in new-born chickens, the observation that pinealectomy leads to an experimental scoliosis in chickens similar to that in humans has led to this procedure being adopted as an attractive model used extensively in experimental research [1, 2, 3, 11, 14, 15, 18, 19, 20, 21, 22, 23, 33, 34]. However, the pathophysiological mechanism of the development of spinal deformity after experimental pinealectomy in young chickens has so far not been clearly explained. As MLT is the main secretory product of the pineal gland, the studies carried out in order to determine the cause of the observed spinal deformities after pinealectomy have focused on MLT. Although this phenomenon has not been observed in humans after surgical pinealectomy [31], dysfunction of specific receptors in the central nervous system due to decreased MLT production was held to be responsible for the ensuing spinal deformity [18, 25, 29, 32].
Recently, some authors have claimed that intraperitoneal administration of MLT or pineal gland transplantation after pinealectomy prevents spinal deformities [15, 23], while others have determined that MLT therapy has no effect on the development or progression of spinal deformities [1, 2, 13]. Such disparity regarding the role of MLT in the development of spinal deformity is confusing and needs further investigation. Therefore, the aim of this study was to investigate the effects of pineal gland transplantation on the development of spinal deformity in young chickens after pinealectomy.
Materials and methods
Experimental animals and groups
The experimental protocol was reviewed and approved by the Ege University School of Medicine Animal Care and Use Committee. A total of 36 Hybro Broiler chickens (2 days of age; 45–60 g) of both sexes were obtained from a local hatchery (Institute of Agricultural Research of Erbeyli). All of the chickens were kept in individual cages under constant laboratory conditions of 18°–21°C room temperature, a 12-h light/dark cycle (6 a.m.–6 p.m.), with cool white fluorescent bulbs. They were given free access to commercial chicken diet, which did not contain MLT and serotonin, and water ad libitum. The chickens were divided randomly into three groups:
Group A: intact control group of 12 chickens, in which no surgical intervention was performed
Group B: pinealectomy group of 12 chickens, in which pinealectomy procedure was performed
Group C: pineal transplantation group of 12 chickens, in which the resected pineal body autografts were implanted into the intramuscular tissue of the trunk after surgical procedure in the pinealectomy group. Using aseptic techniques, the skin overlying the anterior chest wall was opened by a small incision. Then pineal body graft was placed into a pectoral muscle incision using a microforceps. The muscle and skin were sutured in anatomical layers. Care was taken not to damage the gland tissue during the surgery
Pinealectomy and pineal transplantation
The procedure was carried out under the general anesthesia of intraperitoneal sodium pentobarbital (Nembutal sodium, Abbott Laboratories Comp., İstanbul; 40 mg/kg body weight). The part under surgical intervention after shaving was disinfected using polyvidon iyod. In aseptic conditions, a 2-cm midline incision was made at the junction of frontal and parietal bones over the lambdoid fontanel and a skull flap was raised with a scalpel. Then the pineal gland, which lies just beneath the dura mater and between two cerebral hemispheres and cerebellum, was taken out using a microsurgical forceps after cutting it from its pedicle. The skin was sutured with vicryl 6/0. In the pineal transplantation group (group C), pineal body autograft, which was taken out by the pinealectomy procedure, was implanted into the body wall musculature and the skin was sutured quickly.
Radiographic examination
Eight weeks following the pinealectomy procedure, posteroanterior radiographs of the vertebral column of each chicken were taken in a standardized supine position under deep anesthesia using a special table. Care was taken to align the sternum and the spine and to ensure that the pelvic and pectoral girdles were parallel to each other and level.
Cobb angle
At the completion of the study, each radiograph was evaluated for the presence and laterality of scoliosis and the degree of curvature was measured by Cobb's method [7]. In brief, the cephalad and caudal end vertebrae were identified, and lines were drawn parallel to their cephalad and caudal borders, respectively. Additional lines were drawn perpendicular to these lines, and the intersecting angle of the perpendicular lines was measured as the Cobb angle. Scoliosis was defined as a lateral curvature greater than 10°. The chickens in the pinealectomy and transplantation groups were then subdivided into two groups according to whether a scoliotic curve had or had not developed.
Rib-vertebra angle
From the posteroanterior radiographs of all chickens with scoliosis, the involved vertebrae and the apex level of the scoliotic curve were noted for comparison. In addition, in all groups the rib-vertebra angles (RVAs) of the convex and the concave side of the scoliotic curvature were measured on radiographs from level T1 to T8 as described previously by Mehta [24] and Sevastik et al. [28], and the difference between the two, the RVAD (RVA concave–RVA convex), was then calculated for differentiation between progressive and regressive scoliosis. The mean RVAD for each group was calculated, and these values were used for the analysis.
Length of spine
The length of the spine was determined on each radiograph by measuring the straight-line distance, regardless of lateral curve, from the cephalad border of the first thoracic vertebra to the tip of the most caudad vertebra in the tail. The chickens were also weighed while radiographs were taken.
Macroscopic examination
Eight weeks after surgical intervention, all of the chickens were euthanized using an overdose of anesthetic, and their weights were determined as an index of growth. Vertebral columns of the chickens were dissected out along with the thoracic cage. The actual structural changes in the spine were then compared with the plain radiographs. Photographs were taken from different angles whenever the vertebral deformities were characteristic. Photographs and radiographs of normal vertebrae were included for comparison.
Melatonin assay
At the age of 8 weeks, a 5 cc blood sample was taken immediately after death from a peripheral wing vein of each chicken using a 20-G needle at between 1 p.m. and 3 p.m., and put into a glass tube without heparin, and stored at 4°C for 24 h. Then the blood samples were centrifugated at 3000 rpm for 20 min and the collected serum was stored at −80°C until assayed. At the end of the study, MLT concentrations in the collected serum samples were measured by using the enzyme-linked immunosorbent assay (ELISA) method. All samples from each subject were assayed in duplicate to ensure reliability.
Histological evaluation
At the end of the experiment, chickens in each group were killed after radiological examination and collection of blood samples. Their brains were cut into blocks of equal length followed by fixation with 10% buffered formaldehyde. The blocks were embedded in paraffin wax and a representative sagittal section was taken from each. The tissue sections were stained with either hematoxylin and eosin (HE), or Einarson gallocyanin method for light-microscopic observation. The sections were examined to confirm the complete removal of the pineal gland at surgery and the absence of regeneration of the pineal gland.
Data analysis
Data were expressed as arithmetical means ± standard deviations for each group. Comparisons of weights, lengths of spines and serum MLT levels were made by one-way analysis of variance (ANOVA) followed by Duncan's post-hoc test for pairwise comparisons. Degree of scoliotic curve and RVADs values were compared by the Kruskal-Wallis nonparametric test and then Mann-Whitney U test for comparisons between two groups. A P-value of less than 0.05 was considered significant.
Results
One chicken in group B died soon after surgery. It was excluded from the study and replaced by another animal. All other animals showed no evidence of gross neurophysiologic deficit beyond the 1st day of surgery. No postoperative wound infections were noted. In all groups, the chickens outwardly showed normal growth as measured by body weight and length of the spine. No significant differences could be detected in the mean weight of the chickens or the mean length of the spine between all groups (P=0.313 and P=0.090, respectively) (Table 1, Table 2).
Table 1.
Mean weight in the three groups of chickens at 8 weeks of age. No significant differences were found between the groups
| Groups | n | Weight (g) |
|---|---|---|
| Group A | 12 | 2195.83±140.55 |
| Group B | 12 | 2295.83±234.00 |
| Group C | 12 | 2183.33±198.10 |
Table 2.
Mean length of spine in the three groups of chickens at 8 weeks of age. No significant differences were found between the groups
| Groups | n | Length (cm) |
|---|---|---|
| Group A | 12 | 16.76±1.27 |
| Group B | 12 | 15.52±1.45 |
| Group C | 12 | 15.82±1.46 |
Radiographic findings
The radiographs were of good quality and did not present any problem for assessment of development of spinal deformity. Scoliosis developed in 58% of the chickens in group B within 8 weeks after pinealectomy procedure (Table 3). According to the definition of scoliosis described by Cobb [7], none of the animals in group A had spinal deformity (Fig. 1A). The curve severity in group B ranged from 12° to 58° (Fig. 1B). The convexity of the curve was directed to either side with no consistent pattern, and one chicken in this group had double curvature. In group C, with pineal transplantation after pinealectomy, scoliosis developed in 50% of the chickens, and the degree of curve severity ranged from 12° to 46° (Fig. 1C). In groups B and C, the average Cobb angles (18.07°±16.10°, 15.07°±13.80°, respectively) were significantly larger than those found in group A (1.42°±1.56°) (P=0.000 and P=0.001, respectively). However, the difference between the two groups was not statistically significant (P=0.701) (Table 4).
Table 3.
Incidence of scoliotic deformity for each of the experimental groups 8 weeks after surgery
| Groups | n | Chickens with scoliotic deformity (%) |
|---|---|---|
| Group A | 12 | 0 (0) |
| Group B | 12 | 7 (58) |
| Group C | 12 | 6 (50) |
Fig. 1.
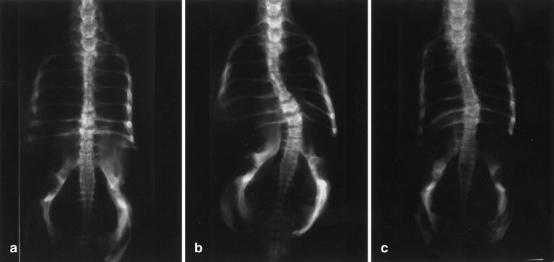
Posteroanterior radiograph of A a straight chicken spine in group A, the control group, B a chicken spine with a severe scoliotic curve in group B (the pinealectomy only group), and C a chicken spine with a mild scoliotic curve in group C (the group with pinealectomy plus implantation of the resected pineal body autografts into the intramuscular tissue of the trunk)
Table 4.
Degree of scoliotic curve in the three groups of chickens at 8 weeks of age
| Groups | n (%) | Cobb angle |
|---|---|---|
| Group A | 0 (0) | 1.42°±1.56° |
| Group B | 6 (50) | 18.07°±16.10°a |
| Group C | 7 (58) | 15.07°13.80°b |
a Significantly different from controls (P=0.000)
b Significantly different from controls (P=0.001)
Their curved spines had the same anatomic features as those found in human scoliosis, particularly regarding the value of RVADs. The average RVADs in groups B and C (11.62°±15.55° and 4.00°±4.88°, respectively) were significantly larger than those found in group A (0.50°±1.45°) (P=0.004 and P=0.016). The average RVAD in group B was larger than that found in group C, but this difference did not reach statistical significance (P=0.264) (Table 5). The apex level of the scoliotic curvature was localized in thoracic (81%), cervical (13%), or cervicothoracic regions (6%) (Table 6).
Table 5.
Rib-vertebra angle differences (RVADs) in the three groups of chickens at 8 weeks of age
| Groups | n (%) | RVAD |
|---|---|---|
| Group A | 0 (0) | 0.50°±1.44° |
| Group B | 6 (50) | 11.62°±15.55°a |
| Group C | 7 (58) | 4.00°±4.88° |
a Significantly different from controls (P<0.05)
Table 6.
Data showing the characteristics of the scoliotic curve in each chicken after pinealectomy
| Group/chicken no. | Curve pattern | Curve severitya | Vertebrae involved | Apex level of curve |
|---|---|---|---|---|
| Group B | ||||
| 2 | Single | 15°L | T4–T8 | T6–T7 |
| 4 | Single | 37°L | T3–L3 | T7 |
| 5 | Single | 20°R | T2–T7 | T4–T5 |
| 6 | Double | 21°L/30°R | T2–T6/T6–L4 | T4/T8 |
| 8 | Single | 58°R | C7–C12 | C9 |
| 9 | Single | 30°L | T5–T8 | T6 |
| 12 | Single | 12°R | T1–T7 | T5 |
| Group C | ||||
| 1 | Single | 12°L | T3–L4 | T7 |
| 3 | Double | 27°R/46°L | T3–T5/T5–L2 | T4/T7–T8 |
| 6 | Single | 16°R | T4–L2 | T8 |
| 7 | Single | 14°R | T2–T6 | T3 |
| 8 | Single | 22°L | T5–T8 | T7 |
| 11 | Double | 38°R/10°L | C5–C11/T1–T5 | C7–C8/T3 |
aR convexity of the curve on the right side, L convexity of the curve on the left side
Macroscopic findings
All chickens in the three groups were examined by visual observation and palpation for the presence of spinal deformity. Seven out of the 12 cases in group B, and 6 out of the 12 cases in group C developed scoliosis. The pattern of the curves in group C was similar to that in group B (Fig. 2). As seen in Table 6, ten of the chickens with scoliosis in groups B and C displayed single curves, and the remaining three cases with scoliosis had double curves. At the age of 8 weeks, double curve was observed in two of six cases in group C. Convexity of the curve was on the right side in 50% of the cases and on the left side in remaining 50%.
Fig. 2.
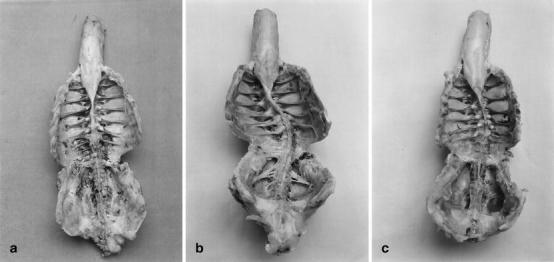
Specimens of chicken spine showing A a straight spine in group A, B severe spinal deformity in group B, and C mild scoliosis in group C
Light-microscopy
Histologic examination of the brains revealed damage to the cerebral cortex at the site of the skull removal and/or pineal gland in some chickens in all groups. In cases in groups B and C, it was confirmed histologically that the pineal gland had been removed at surgery and that no extraneous tissue had been left behind or had regenerated (Fig. 3A,B).
Fig. 3.
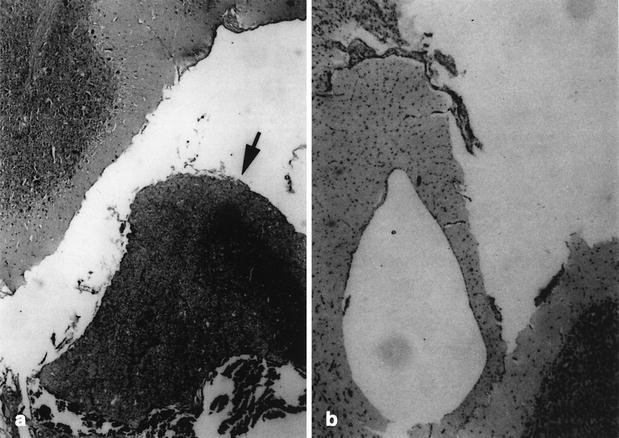
Histological examination of a sagittal section of brain in A the intact control chickens (group A), and B the pinealectomy chickens (groups B and C). H&E stain; original magnification x126. Note brain in normal and pineal gland (arrow)
Serum melatonin level
The average serum MLT levels from the chickens subjected to pinealectomy in group B and pineal transplantation in group C were low (9.17 ±2.00 pg/ml, 10.07±3.83 pg/ml, respectively). In contrast, the average value for group A was significantly higher than those in groups B and C (23.19±2.33 pg/ml, P < 0.05) (Table 7).
Table 7.
Serum melatonin levels in the three groups of chickens at 8 weeks after surgery
| Groups | n | Serum melatonin (pg/ml) |
|---|---|---|
| Group A | 12 | 23.19±2.33 |
| Group B | 12 | 9.17±2.00a |
| Group C | 12 | 10.07±3.83b |
a Significantly different from controls (P<0.05)
b Significantly different from controls (P<0.05)
Discussion
The results of this experiment demonstrate that when pineal body autograft was placed into intramuscular tissue of the trunk following pinealectomy done 2 days after hatching, it has no effect on either the serum MLT concentrations or the development of spinal deformity in chickens. Recently, Bagnall et al. [2] also reported that the production of a scoliotic curvature in young chickens was not affected by MLT administration after pinealectomy. These results are in sharp contrast to those reported by Machida et al. [19, 21], who designed a similar study and found that pineal gland transplantation and MLT therapy following pinealectomy procedure prevented the development of spinal deformity. The reason for the differences between these two studies is not obvious, and such a confusing disparity warrants additional study.
In the chicken, the pineal gland is a small, median, conical, dorsally-directed "pineal bulb" attached by a long "pineal stalk" to the posterior aspect of the third ventricle, lying in the space between the cerebral hemispheres and the cerebellum deep to the skull [16]. A few blood vessels and nerve fibers supply the gland via the pineal stalk. The nerve fibers arising in the superior cervical ganglia end directly in relation to the pinealocytes and are thought to influence the secretion of MLT. Cutting the pineal stalk during the pinealectomy procedure is always accompanied by a significant decrease in serum MLT levels as a result of nonfunctional pineal gland [3]. It has been shown that the pineal gland may continue to secrete MLT for a few days because the pineal gland in chickens has an endogenous rhythm [10]. Thus, it was expected that transplantation of the pineal gland into the body wall musculature after pinealectomy procedure would not result in continued functioning of the pineal gland in chickens. The results of this current experiment clearly show that pineal gland becomes nonfunctional with respect to serum MLT after pinealectomy with transplantation, and that transplantation of the pineal gland does not cause a reduction in the development of spinal deformity. The results of our study stand in sharp contrast to the results of Machida et al. [19], who demonstrated that transplantation of the pineal gland after pinealectomy in young chickens leads to re-establishment of normal serum MLT levels and a significant reduction of scoliosis. It is unclear why the pineal gland should remain functional following transplantation, when its vascular and neural supply has been severed. Indeed, there is only one possible explanation for this observation: the presence of extrapineal tissues as a possible source of MLT [17]. Theoretically, it is possible that these tissues compensatorily increase their MLT production following pinealectomy. In the present study, it would be important to know the morphological status of the biologically inert gland in pinealectomized chickens: the survival, revascularization, neural reinnervation, and structural changes.
In the current study, the chickens in the control group A exhibited no spinal deformity. Conversely, the results show that scoliosis was observed in 58% and 50% of groups B and C, respectively. Although Machida et al. [19, 20, 21, 23] have reported that 100% of their pinealectomized chickens developed a scoliotic curve, these results have not been confirmed in other studies [1, 2, 3, 9, 14, 30, 33, 34]. Recently, a 52% incidence of scoliosis after pinealectomy was reported by Wang et al. [34], and this is confirmed by the results of our current study. A review of the literature (Table 8) shows that, among the limited number of experimental studies of scoliotic deformity development following pinealectomy in chickens that have been published to our knowledge (including ours), all of them, except Machida et al. [19, 20, 21, 23] and Coillard and Rivard [9], demonstrated that scoliosis did not develop in all cases after pinealectomy in young chickens (2–3 days after hatching), even though serum MLT levels were significantly low [2, 3, 14, 33, 34]. Although there was no major difference in terms of definition of scoliosis, species of pinealectomized chicken or surgical pinealectomy technique among the studies, the observation period in these series ranged from 5 weeks to 32 weeks after pinealectomy, and the rate of experimentally induced scoliosis ranged from 52% to 100% [2, 3, 9, 14, 19, 20, 21, 23, 33, 34]. The observation period was 12 weeks in the studies reported by Machida et al. [19, 20, 21, 23], but in those reported by Wang et al. [33, 34], Bagnall et al. [2], and Beuerlein et al. [3], it was only 5 weeks. With the exception of the study by Kanemura et al. [14], a 100% incidence of scoliotic deformity has been reported in all other studies that had an observation period of at least 12 weeks. For this reason, it seems likely that a possible explanation for such a discrepancy in the incidence of scoliosis among the chickens subjected to pinealectomy in the studies may be related to differences in the observation periods. Accordingly, an investigational work on the natural course of scoliosis in pinealectomized chickens by Kanemura et al. [14] led to the conclusion that the scoliotic curve progresses as the chickens grow older.
Table 8.
Summary of experimental studies of scoliosis following pinealectomy in chickens reported in the literature
| Authors | Reference no. | Year | Age after hatching | Species of chicken | Observation period | Incidence of scoliosis (%) |
|---|---|---|---|---|---|---|
| Machida et al. | [19] | 1993 | Not stated | White leghorn | 12 weeks | 100 |
| Machida et al. | [20] | 1994 | 2 days | White leghorn | 12 weeks | 82–100a |
| Machida et al. | [21] | 1995 | 3 days | White leghorn | 12 weeks | 100 |
| Coillard & Rivard | [9] | 1996 | Shortly | Hybro broiler | 32 weeks | 100 |
| Kanemura et al. | [14] | 1997 | 3 days | Not stated | 16 weeks | 85 |
| Machida et al. | [23] | 1997 | 2 days | White leghorn | 12 weeks | 100 |
| Wang et al. | [33] | 1997 | 3 days | White leghorn | 6 weeks | 58 |
| Wang et al. | [34] | 1998 | 3 days | White leghorn | 5 weeks | 52 |
| Bagnall et al. | [2] | 1999 | 3 days | Mountain hubbard | 5 weeks | 61 |
| Beuerlein et al. | [3] | 2001 | 3 days | White leghorn | 5 weeks | 63 |
| Present study | 2003 | 2 days | Hybro broiler | 8 weeks | 58 |
a 82% for male chickens and 100% for female chickens
Unfortunately, there is no conclusive evidence regarding the natural course of scoliotic deformity in chickens in the present study. However, it seems to be logical to measure the RVADs using the methodology described by Mehta [24] for predicting the progression after pinealectomy. Thus, we could predict that for a mild scoliotic curvature of less than 10° at first presentation, the initial curve would show progression to severe scoliosis exceeding 10° towards the end of the observation period. In all experimental studies in Table 8, a lateral curvature with a Cobb angle greater than 10° was defined as scoliosis at some stage during the experiment. To our knowledge, the current study is the first investigation regarding RVADs as an early indicator of future scoliotic deformity development in the pinealectomized chickens in the literature available.
On the other hand, the current study has certain limitations. One of them was the absence of information about other forms of spinal deformity such as lordosis and kyphosis, because only posteroanterior radiographs of the spine were taken in all chickens. Obviously, it would have been necessary to take lateral radiographs as well for evaluation of such a deformity. Another criticism was the need for a longer observation period—at least 3–6 months—for assessment of the natural course of scoliosis in chickens. Also, inclusion of cases with a Cobb angle smaller than 10° into scoliosis group would provide some data regarding its natural course. Although the common "bipedal" feature of chickens and humans is critical for the development of scoliotic curve, there are obvious anatomical and biomechanical differences between avian and human spines. In addition, it is also stated that "spontaneous" scoliosis can develop in chickens at a rate as high as 30% [26]. Therefore, it seems clear that the results of experimental studies involving chickens cannot be directly extrapolated to clinical studies in human beings. More studies on the role of MLT in the development of scoliosis after pinealectomy in chickens are needed.
From the current results, it can be concluded that:
The pinealectomy procedure may produce scoliotic deformity in young chickens, but low serum MLT levels do not always result in scoliosis development.
A possible explanation for the low rate of scoliosis incidence in our experiment at the end of the 8-week observation period may be shortness of the time after pinealectomy.
The morphological characteristics of scoliotic deformity in melatonin-deficient young chickens are similar to those of idiopathic scoliosis in humans.
Intramuscular autografting of the pineal body after pinealectomy in new-born chickens does not restore plasma MLT levels.
Pineal gland transplantation for substitution of MLT has no effect on the development of scoliotic deformity in the pinealectomized chickens over an 8-week follow-up.
Therefore, the role of MLT in the development of spinal deformity in chickens after pinealectomy remains controversial.
However, it is reasonable to believe that the development of scoliosis after pinealectomy in chickens is multifactorial in origin, rather than arising from a single cause. It is possible that MLT has an effect on the brainstem and spinal cord in maintaining spinal balance and equilibrium, because its receptors are widespread in the chicken brainstem, cerebellum and spinal cord [12, 25, 29, 32]. At present, the mechanisms by which pinealectomy procedure in young chickens consistently results in the development of spinal deformity are still not understood, and further studies are needed to clarify the role of other factors behind this phenomenon. In future, it will be interesting to evaluate the features of the transplanted gland by light- and electron-microscopic studies as well as by immunohistochemical analysis.
Acknowledgements
We wish to thank Ogün Beyazıtlı, Seda Çoşkun and Süleyman Ögün for skilful technical assistance and Hatice Üstün for the statistical analysis. The authors also gratefully acknowledge the secretarial support by Pınar Yüzüak.
Footnotes
The authors made substantial contributions to the following tasks of research: design (M.T., A.U., M.B.); provision of resources (M.T., M.B., M.E.Y.); collection of data (M.T., Ç.Y., A.U., M.B.); laboratory analysis and interpretation of data (M.T., Ç.Y., A.U., M.B., M.E.Y.); writing and revision of paper (M.T., Ç.Y., A.U., M.E.Y.); study supervision (M.T., M.E.Y). The views expressed herein are those of the authors and not necessarily their institutions or sources of support. There are no potential conflicts of interest.
This study was presented in part at the 37th National Annual Neurological Congress, Antalya, 31 October–4 November, 2001
References
- 1.Bagnall Spine. 1996;21:1974. doi: 10.1097/00007632-199609010-00006. [DOI] [PubMed] [Google Scholar]
- 2.Bagnall J Bone Joint Surg Am. 1999;81:191. doi: 10.2106/00004623-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Beuerlein Spine. 2001;26:237. doi: 10.1097/00007632-200102010-00007. [DOI] [PubMed] [Google Scholar]
- 4.Beyer Biochem Pharmacol. 1999;56:1265. doi: 10.1016/S0006-2952(98)00180-4. [DOI] [PubMed] [Google Scholar]
- 5.Brodner J Bone Joint Surg Br. 2000;82:399. doi: 10.1302/0301-620x.82b3.10208. [DOI] [PubMed] [Google Scholar]
- 6.Brzezinski N Engl J Med. 1997;336:186. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- 7.Cobb Am Acad Orthop Surg. 1948;5:261. [Google Scholar]
- 8.Cohen Ann Intern Med. 1964;61:1144. doi: 10.7326/0003-4819-61-6-1144. [DOI] [PubMed] [Google Scholar]
- 9.Coillard Eur Spine J. 1996;5:91. doi: 10.1007/BF00298387. [DOI] [PubMed] [Google Scholar]
- 10.Deguchi J Spinal Disord. 1996;9:207. [PubMed] [Google Scholar]
- 11.Dubousset Orthop Trans. 1983;7:7. [Google Scholar]
- 12.Fagan Spine. 1998;23:41. doi: 10.1097/00007632-199801010-00009. [DOI] [PubMed] [Google Scholar]
- 13.Hilibrand Spine. 1996;21:1140. doi: 10.1097/00007632-199605150-00004. [DOI] [PubMed] [Google Scholar]
- 14.Kanemura Spine. 1997;22:1563. doi: 10.1097/00007632-199707150-00006. [DOI] [PubMed] [Google Scholar]
- 15.Kimura Spine. 1993;18:1609. doi: 10.1097/00007632-199309000-00007. [DOI] [PubMed] [Google Scholar]
- 16.King McLelland. 1984;J:their. [Google Scholar]
- 17.Kvetnoy Histochem J. 1999;31:1. doi: 10.1023/A:1003431122334. [DOI] [PubMed] [Google Scholar]
- 18.Machida Spine. 1999;24:2576. doi: 10.1097/00007632-199912150-00004. [DOI] [PubMed] [Google Scholar]
- 19.Machida Spine. 1993;18:1609. doi: 10.1097/00007632-199309000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Machida J Pediatr Orthop. 1994;14:329. doi: 10.1097/01241398-199405000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Machida J Bone Joint Surg Br. 1995;77:134. [PubMed] [Google Scholar]
- 22.Machida Spine. 1996;21:1147. doi: 10.1097/00007632-199605150-00005. [DOI] [PubMed] [Google Scholar]
- 23.Machida Spine. 1997;22:1297. doi: 10.1097/00007632-199706150-00004. [DOI] [PubMed] [Google Scholar]
- 24.Mehta J Bone Joint Surg Br. 1972;54:230. [PubMed] [Google Scholar]
- 25.Pang Biol Signals. 1997;6:272. [PubMed] [Google Scholar]
- 26.Rigdon Avian Dis. 1968;12:530. [PubMed] [Google Scholar]
- 27.Sawaya R, Hawley DK, Tobler WD, Tew JM Jr, Chambers AA (1990) Pineal and third ventricle tumors. In: Youmans JR (ed) Neurological surgery, 3rd edn. WB Saunders, Philadelphia, pp 3171–3203
- 28.Sevastik Eur Spine J. 1997;6:84. doi: 10.1007/BF01358737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SiuciakJ Neurosci 19911118552045890 [Google Scholar]
- 30.Thillard Association des Anatomistes. 1959;46:22. [Google Scholar]
- 31.Vorkapic Neurosurgery. 1987;21:817. doi: 10.1227/00006123-198712000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Wan Neurosci Lett. 1994;180:253. doi: 10.1016/0304-3940(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 33.Wang Spine. 1997;22:2626. doi: 10.1097/00007632-199711150-00010. [DOI] [PubMed] [Google Scholar]
- 34.Wang Spine. 1998;23:2377. doi: 10.1097/00007632-199811150-00002. [DOI] [PubMed] [Google Scholar]
- 35.Wurtman RJ, Cardinal DP (1974) The pineal organ. In: Williams RH (ed) Textbook of endocrinology. WB Saunders, Philadelphia, pp 832–840


