Abstract
Congenital scoliosis is the most frequent congenital deformity of the spine. Congenital curvatures are due to anomalous development of the vertebrae (failure of formation and/or segmentation). Congenital scoliosis is believed to be related to an insult to the fetus during spine embryological development, and associated malformations (heart, spinal cord, kidney...) are frequently observed. A perfect understanding of the natural history of the deformity and the treatment principles will allow best management of these complex spine deformities. New imaging techniques like three-dimensional computed tomography (CT) and magnetic resonance imaging (MRI) are important tools for analyzing the underlying deformity and understanding the evolution of the complex deformities. The mainstay of treatment is either observation or, in case of curve progression (>10°/year), surgery. Different surgeries are described with two main principles: (1) prophylactic surgeries like hemiepiphysiodesis or in situ fusions that will prevent worsening or allow progressive correction over time, and (2) corrective surgeries, with spinal fusion with or without spinal resection. Exceptional procedures (e.g. spinal column resection or halo distraction) can be attempted in cases of very severe deformity. Congenital curves must be carefully observed to choose the least invasive procedure at the right time and to minimize spinal cord risks.
Keywords: Congenital scoliosis, Spinal deformity, Spine surgery
Introduction
Congenital scoliosis is the most frequent congenital deformity of the spine, the two others being congenital kyphosis and congenital lordosis. Congenital curvatures are due to anomalous development of the vertebrae (failure of formation and/or segmentation).
In most cases congenital scolioses are non-hereditary [29, 32]. Congenital scoliosis is believed to be related to an insult to the fetus during spine embryological development (between the 5th and 8th week of gestation). This is why other malformations such as congenital heart disease, spinal cord dysraphism, or kidney malformations are frequently associated. Only cases with syndromic associations (Jarcho-Levin, spondylocostal dysplasia) or multiple defects of segmentation can have a hereditary factor [22]. A perfect understanding of the natural history of the deformity and the treatment principles will allow best management of these complex spine deformities. Statistically, 25% of curves are non-progressive, 25% mildly progressive and 50% highly progressive and will require treatment [21, 31, 32, 35]. The mainstay of treatment is either observation or surgery.
Circumstances of discovery
Quite often, the diagnostic of the spine deformity is made at the time of the prenatal ultrasound [3]. In most cases the exact diagnosis of the deformity cannot be made. It is, however, essential that the radiologist who performs the ultrasound look for any other associated conditions such as spina-bifida, or heart, urogenital or other syndromic malformations at this time. Prenatal counseling and awareness of the overall prognosis of these kinds of deformities is essential to provide appropriate information to the parents.
Incidental discovery on routine X-rays (chest X-rays for instance) done for any other reason such as congenital heart disease, respiratory problems, or abdominal pain should not be overlooked, as they may later provide an essential element in assessing progression of the deformity.
The child will otherwise be seen for the discovery of a spine deformity picked up by the family or their pediatrician (lump in the back discovered incidentally). Obviously, a hairy patch or a skin hemangioma in the midline, or even a sacral dimple must raise the suspicion of an underlying congenital malformation. Neurologic findings such as a foot malformation or a leg asymmetry, or urinary symptoms and an unusual and rigid curve will direct the astute physician towards the spine looking for a malformation.
In extreme cases, congenital scoliosis is only discovered at the time of the surgical procedure (of what was thought to be an idiopathic scoliosis), as it may not have been visible on the radiographs due to the rotation of the vertebrae.
Clinical examination and necessary work-up
The evaluation of the patient follows the rules of spinal deformity examination. An assessment is made of the balance of the trunk, using a plumb-line dropped from C7 and the skull, and of the balance of the shoulders, using a spirit level. The rigidity of the curve is assessed, and the rib hump measured. Clinical digitalized photographs will be taken, as they best describe the patient's clinical presentation.
Associated malformations must be looked for clinically and with an appropriate work-up: one should look for any foot or leg asymmetry, any craniofacial malformation, a Klippel-Feil web neck, and cardiac and urinary malformations, and one should naturally perform a very thorough neurologic examination. The incidence of associated malformation has been reported to be as high as 25% for urologic conditions, 10% for cardiac conditions, and 28–40% for neuraxis anomalies [2, 6, 33].
The appropriate work-up will therefore include standing whole spine X-rays, and cervical spine X-rays to rule out a Klippel-Feil or a cervical hemivertebra. Spot views of the malformation may be necessary. Chest cage X-rays will be required in the case of a thoracic curve to look for rib synostosis, which may behave as a bar if it is close to the spine. Ultrasound may pick up kidney abnormalities, which are associated in 20% of cases of congenital scoliosis [20]. A cardiac assessment is also required by cardiac echography or by the cardiologist, as congenital scoliosis has a 12% incidence of associated cardiac malformation [2]. Magnetic resonance imaging (MRI) will find spinal cord anomalies in 40% of the cases. It can be a tethered cord, a diastematomyelia, a syrinx, or a Chiari malformation. MRI is mandatory before any surgical intervention.
Classification and prognosis
Full-body-width postero-anterior (PA) and lateral X-rays are essential. The best X-rays are usually ones taken at birth, and one should track them down if they are available. After 1 year of age, these X-rays should be taken standing, with the legs in extension and the pelvis level, to compensate for any leg length discrepancy. The Cobb angle will be measured from end-plate to end-plate or, when not feasible, one should use the pedicle lines. It is essential that the same landmarks be used during the whole follow-up period. One may therefore have to calculate several Cobb angles (the one within the deformation area and the one of the overall curve). It is accepted that in congenital scoliosis a worsening of the Cobb angle of at least 10° is sufficiently significant to be termed an aggravation [19]. Tomographs are classic for showing a bony bar, but not very helpful for the experienced surgeon. Computed tomography (CT) with thin slices and with reconstruction is useful in very complex deformities. MRI with cartilage sequences provides the best quality pictures of the cartilage end-plates, possibly giving the best information on growth potential (Fig. 1).
Fig. 1.
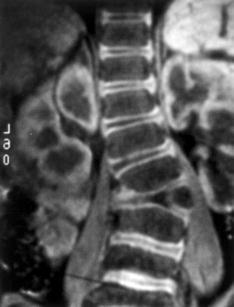
Magnetic resonance imaging with cartilage sequence of a fully segmented hemivertebra
Congenital scoliosis can be classified into defect of formation or defect of segmentation, yet most malformations have combined features [22]. The potential of worsening of the curve is in fact related to the asymmetry in growth potential on the convex and concave sides of the curve, and a grading of curves from worse to best prognosis would therefore look as follows:
Fully segmented hemivertebra (two growth plates) with a controlateral bar: they sometimes get worse at a rate of 10°/year [21]
Unilateral unsegmented bar
Two consecutive fully segmented hemivertebrae
Fully segmented hemivertebra
Semisegmented hemivertebra
Wedge vertebra (corresponding to a partial failure of formation)
Incarcerated hemivertebra (these are smaller and they induce little deformity as the hemivertebra sits in a non-growing block where the hemivertebra has created a niche; the alignment of the pedicles usually remains straight)
The location of the curve has an impact on the overall progression: upper thoracic curves tend to progress less than thoracolumbar and lumbar curves. Yet though these upper thoracic curves seldom reach 30°, they may cause significant shoulder imbalance and require treatment. Mediothoracic curves (apex at T5–T7) may induce progressive lower thoracic or lumbar curves that may need to be included in the fusion when they become bigger and structural. It is therefore important to intervene before such changes happen. Low lumbar curves may induce an oblique take-off of the spine, with pelvic obliquity and trunk imbalance. In cases of posterior wedge vertebra and/or dislocation, development of the vertebra can also directly damage the neurologic elements by growing in the canal.
The diagnosis of progression will be based on serial clinical examinations (every 6–9 months from birth to 5 years of age, every year from 5 to 10, and every 6 months from puberty to the end of skeletal maturity). Clinical photographs of the patient are the best monitoring tools. On the radiographs, which will always be compared with the initial radiographs, one can measure the Cobb angle, assess the rotation, and look at the rib vertebral angle (ribs becoming more vertical) and the compensatory curves. Sometimes the Cobb angle does not change but the curve worsens, and this is picked up by an increased shoulder imbalance, trunk shift or a worsening of the compensatory curve. Progression occurs naturally due to the persisting growth on the convex side of the deformity.
Treatment
Treatment of congenital scoliosis will consist in either observation of the curve or surgery, or, rarely, bracing. Observation should be applied only for non-progressive curves with a balanced spine:
Bracing in most instances is totally inefficient in congenital scoliosis. It may be indicated for long flexible curves or to control the compensatory lumbar curve or help rebalancing the spine, or it may be used after an operation, for instance, until the fusion is solid.
Surgery
Surgery is the mainstay of care. A variety of procedures are used to address different problems.
Prophylactic surgery
By prophylactic surgery we mean surgery that will prevent further worsening or even allow progressive correction over time. These operations refer to in situ fusion and hemiepiphysiodesis.
In situ fusion can be done with a single posterior fusion with or without instrumentation or with an anterior fusion, or even a 360° fusion. These operations will be done once the three-dimensional aspects of the deformity have been understood, and aim to achieve a halt in the progression of the deformity. However, the compensatory curve above the fused area may still progress on its own after such operations. Some corrections of the so-called fusions can be achieved if one uses a corrective cast postoperatively.
Hemiepiphysiodesis tends to achieve progressive correction over time, taking advantage of the intact growth plates on the concave side of the deformity. In most cases it requires a front and back approach to the spine: anteriorly, only the outer one-third of the disc space and one-third of the end-plates are removed. The fusion is carried out with an inlay segment of the rib, for instance. Posteriorly, only the convex side is approached and fused. The patient is then immobilized in a cast in the position of maximum correction to take advantage of the flexibility of the curve. These operations have to be done ideally before the age of 5 to take advantage of the growth potential of the spine to allow a progressive correction of the deformity. The hemiepiphysiodesis requires intact concave growth potentials and proper planning of the extent of the fusion to allow for progressive correction. The results are, however, somewhat unpredictable [10, 11, 15, 30, 34], and these procedures are therefore limited to young patients (under 5 years of age) and to curves of less than 50° (Fig. 2). They should not be carried out if there is a kyphosis component to the deformity. A very careful follow-up is necessary, as progression of the deformity can still occur during the adolescent growth spurt. The hemiepiphysiodesis can be performed through a mini-thoracotomy (trans- or retropleural) or through a thoracoscopic approach, or even through the pedicle [14, 25].
Fig. 2.
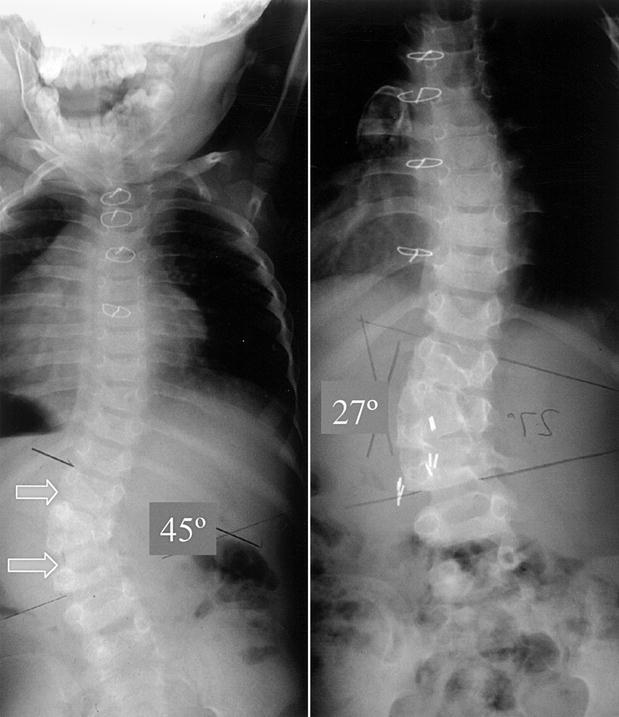
Result of a hemiepiphysiodesis for a semisegmented left second lumbar hemivertebra. Left: progressive 45° lumbar curve at the age of 3 years. Right: at the age of 6 years, the curve has spontaneously corrected to 27° after a T12–L3 hemiepiphysiodesis
Corrective surgery without resection
Posterior spine fusion without instrumentation and correction with a cast can be indicated in young children, but the lack of anterior fusion exposes the spine to the crankshaft phenomenon if the anterior growth plates overcome the posterior fusion.
Posterior spine fusion with instrumentation is indicated in older teenagers, where there is no risk of crankshafting (Fig. 3), but there is a risk of neurological complications if too much distraction is applied [33]. Besides, the amount of correction obtained can lead to a gap in front of the spine, and further pseudarthrosis may develop, especially if there is some kyphosis.
Fig. 3.
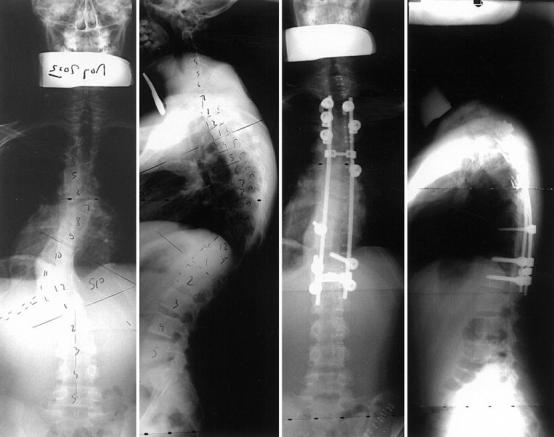
Progressive kyphoscoliosis in a 15-year-old boy due to a hemivertebra at T11 and T7. Strong posterior fusion with iliac crest bone and instrumentation achieved perfect balance of the spine through the mobile segments of the spine
Anterior and posterior spine fusion with discectomies and instrumentation can achieve a significant correction in the mobile segments of the spine. However, the danger is over-correction, with distraction of the curve and neurologic complications. In such cases the distraction should never be done first. The compression rod should be inserted first and then only minimal distraction applied on the concave rod. The use of spinal cord monitoring and an immediate wake-up test after correction is here, more than anywhere else, mandatory. Neurologic monitoring can never be emphasized enough during such corrections.
Anterior stabilization of the spine with a strut graft done through a convex, or preferably concave (for biomechanical reasons), approach should always be considered when there is a kyphotic component to the deformity (Fig. 4). One can use a rib or fibula or even a tibial strut autograft, or even a vascularised rib [23].
Fig. 4. a,b.
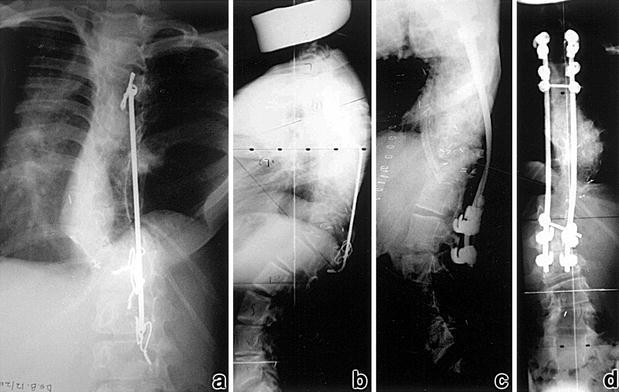
Progression of an already operated congenital scoliosis with kyphosis (note the chest wall hypoplasia). c,d Posterior fusion with a partial correction of the curve and anterior fusion with a rib used as a strut to bridge the kyphotic deformity
Corrective surgery with osteotomy
Osteotomy of the bar and subsequent corrective instrumentation is another possible surgical treatment. CT scan with thin slices is essential in planning this surgery, which is done through a single posterior approach or a combined approach. The inherent neurologic risks of such techniques must be well understood before undertaking such procedures.
Corrective surgeries with spine shortening
Hemivertebra resection
Hemivertebra resection is done either through a posterior approach only or through a sequential or simultaneous front and back approach [4, 7, 13, 16, 17, 18, 24, 26, 27]. These procedures usually provide an average of 25°–30° of correction, with some correction of the associated kyphosis. The correction is usually obtained with a pedicle screw rod system.
Whether one opts for a posterior only approach or a 360° sequential depends on the location of the hemivertebra, its type, whether it is segmented or not and familiarity with the technique. The best indications of hemivertebra resection are the lumbosacral hemivertebra or the hemivertebra situated below the spinal cord and responsible for an oblique take-off with pelvic obliquity (Fig. 5). In the thoracic spine, these resections are definitely more dangerous and should only be performed by experienced spine surgeons. Recent publications tend to show that hemivertebra resection is safe even in the thoracic spine, provided it is performed by experienced surgeons [13]. The classic indications for a hemiresection are, of course, rigid decompensated spinal deformities where other procedures will not achieve proper realignment. In small patients, 4.5-mm AO screws inserted in the pedicles with a tension band system made of simple wires will ensure perfect correction (Fig. 5). In other patients, supra or infralaminar hooks will allow the correction, which will be maintained through use of a cast or brace for 3–4 months. In older patients a classic pedicle screw rod system will be indicated.
Fig. 5.
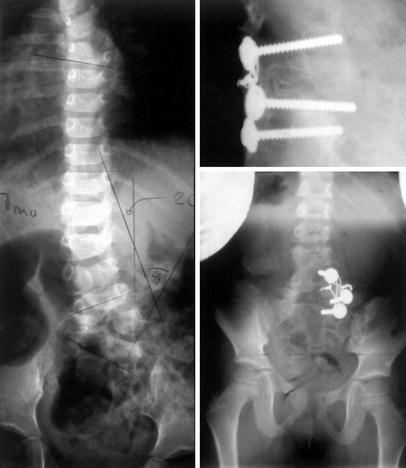
Lumbosacral hemivertebra with trunk imbalance in a 3-year-old child. Hemiresection through a single posterior approach and a correction with pedicle screws and tension band wiring
Spinal column resection
In very complex spinal deformities the only way to rebalance the spine may be through a spinal column resection with shortening of the spinal column (Fig. 6). This was described by Bradford and Tribus, and consists in an anterior approach where one or several vertebrae are removed after a decorticated osteoperiosteal flap has been elevated [5]. The vertebral body/bodies is/are removed down to the dura, the convex pedicles are removed, and as much as possible of the concave pedicles are removed. The posterior surgery, done in the same sitting or a few days later, will consist in removing the corresponding posterior laminae and the rest of the concave pedicles. The spine will be corrected at the same time as the shortening is carried out. Very careful monitoring of the neurologic function is mandatory during these exceptional procedures.
Fig. 6. a–c.
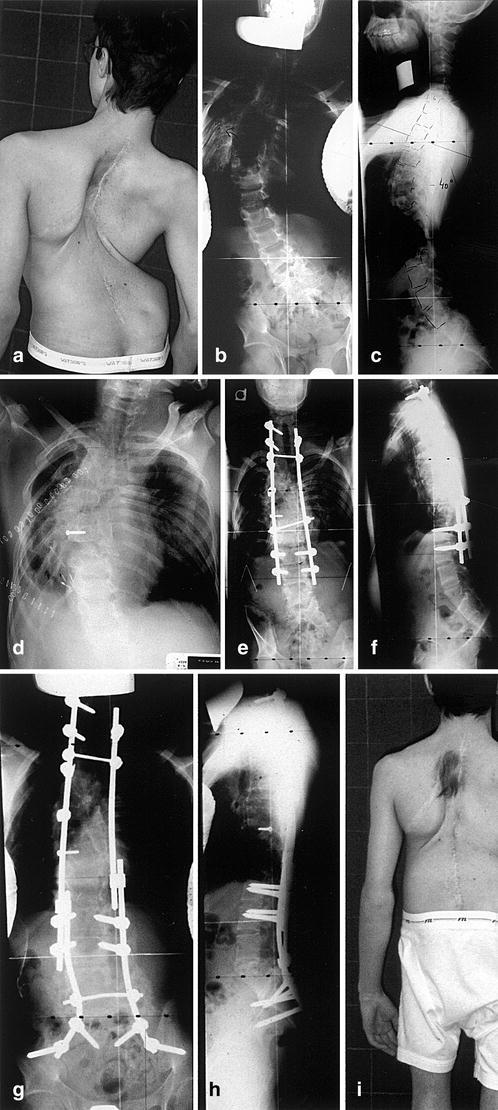
Lordotic evolution of a multioperated congenital scoliosis with thoracic diastematomyelia. Note the thoracic and lumbosacral malformation. d–f The first stage consisted in anterior resection of T9. The second stage consisted in posterior resection of T9 lamina associated with shortening of the spine and correction of the lordosis. g–i The final stage, performed 12 months later, was a posterior lumbosacral hemiresection to achieve final balance
Miscellaneous
The use of Halo traction should be exceptional in congenital scoliosis, and it may be dangerous for neurologic function. Its use is formally contraindicated if there is a rigid acute component of kyphosis associated with the scoliosis. However, in selected cases (Fig. 7) it may be a helpful adjunct, especially in order to prepare the patient for surgery in cases of severe respiratory compromise or in between staged surgery [1, 12, 28, 33].
Fig. 7. a.
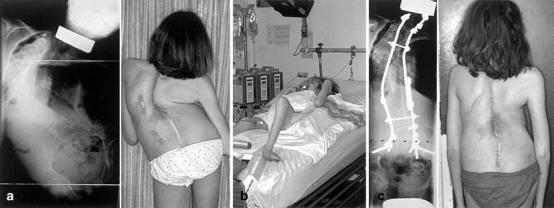
Congenital scoliosis previously multioperated. Vital capacity =500 cc. b Treatment with halogravity traction for 6 weeks followed by posterior osteotomies and halofemoral traction for 3 weeks and posterior fusion with tibial graft. c Result at 3 years' follow-up. Vital capacity=1200 cc
The rib expander—the titanium rib expansion project developed in San Antonio by Campbell—will allow some spine growth as well as chest and lung expansion if carried out before the age of 8, to recruit more pulmonary alveoli [8]. Its best indications are in cases of congenital scoliosis associated with fused ribs and/or patients with thoracic insufficiency syndrome and/or chest hypoplasia.
Subcutaneous rods without fusion and subsequent lengthening may play a role in maintaining the growth of the spine in very young children, but these procedures do not address the area where the malformation of the spine is. They may be combined with convex growth arrest [9]. They expose the patient to multiple lengthening operations and carry a significant risk of complications, mostly infections or instrument complications.
Conclusion
The main goal in congenital scoliosis is not to perform extraordinary operations, but to pick up the curves at early stages where a prophylactic treatment can be achieved without risk to the spinal cord.
One should always be extremely careful with all the distraction systems that can lead to neurologic complications. Shortening procedures will, therefore, often be preferred. The most common mistakes in congenital deformities are:
Delaying treatment in a hope of achieving further growth
Fusing only the pathologic segment of the deformity, whereas all the curve has to be fused in general
Carrying out only a posterior fusion, where a 360° procedure is necessary
Several operations may be necessary during growth, especially if the growth potentials are not balanced early, if the child is left with an imbalance or if the compensatory curves above or below the malformations become structural and worsen on their own.
References
- 1.Arlet Eur Spine J. 1999;8:329. doi: 10.1007/s005860050182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beals Spine. 1993;18:1329. doi: 10.1097/00007632-199308000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Benacerraf J Ultrasound Med. 1986;5:257. doi: 10.7863/jum.1986.5.5.257. [DOI] [PubMed] [Google Scholar]
- 4.Bradford J Bone Joint Surg Am. 1990;72:536. [PubMed] [Google Scholar]
- 5.Bradford Spine. 1997;22:1590. doi: 10.1097/00007632-199707150-00013. [DOI] [PubMed] [Google Scholar]
- 6.Bradford J Pediatr Orthop. 1991;11:36. doi: 10.1097/01241398-199101000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Callahan J Pediatr Orthop J. 1997;17:96. doi: 10.1097/00004694-199701000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Campbell RM, Vocke AK (2001) Growth of the thoracic spine in congenital scoliosis after expansion thoracoplasty. Scoliosis Research Society 36th annual meeting. Cleveland, Ohio [DOI] [PubMed]
- 9.Cheung Spine. 2002;27:748. doi: 10.1097/00007632-200204010-00012. [DOI] [PubMed] [Google Scholar]
- 10.Chirpaz-Cerbat Eur J Pediatr Surg. 1993;3:144. doi: 10.1055/s-2008-1063531. [DOI] [PubMed] [Google Scholar]
- 11.Dubousset J, Katti E, Seringe R (1993) Epiphysiodesis of the spine in young children for congenital spinal deformations. J Pediatr Orthop Part B 1–292:123–130
- 12.Hefti Orthopade. 2002;31:34. doi: 10.1007/s132-002-8272-y. [DOI] [PubMed] [Google Scholar]
- 13.Holte J Bone Joint Surg Am. 1995;77:159. doi: 10.2106/00004623-199502000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Keller Spine. 1994;19:1933. doi: 10.1097/00007632-199409000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Kieffer Eur Spine J. 1994;3:120. doi: 10.1007/BF02221453. [DOI] [PubMed] [Google Scholar]
- 16.Klemme J Pediatr Orthop. 2001;21:761. doi: 10.1097/00004694-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 17.LazarClin Orthop 19993647610416395 [Google Scholar]
- 18.Leatherman, J Bone Joint Surg Br. 1978;61:324. doi: 10.1302/0301-620X.61B3.479255. [DOI] [PubMed] [Google Scholar]
- 19.Loder J Bone Joint Surg Br. 1995;77:768. [PubMed] [Google Scholar]
- 20.MacEwen J Bone Joint Surg Am. 1982;54:1451. [PubMed] [Google Scholar]
- 21.McMaster Spine. 1998;23:998. doi: 10.1097/00007632-199805010-00007. [DOI] [PubMed] [Google Scholar]
- 22.McMaster J Bone Joint Surg Am. 1982;64:1128. [PubMed] [Google Scholar]
- 23.McMaster Spine. 2001;26:2146. doi: 10.1097/00007632-200110010-00021. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura Spine. 2002;27:110. doi: 10.1097/00007632-200201010-00026. [DOI] [PubMed] [Google Scholar]
- 25.Rothenberg J Pediatr Surg. 1998;33:1168. doi: 10.1016/s0022-3468(98)90553-x. [DOI] [PubMed] [Google Scholar]
- 26.Ruf Spine. 2002;27:1116. doi: 10.1097/00007632-200205150-00020. [DOI] [PubMed] [Google Scholar]
- 27.Shono Spine 1. 2001;26:752. doi: 10.1097/00007632-200104010-00011. [DOI] [PubMed] [Google Scholar]
- 28.Sink J Pediatr Orthop. 2001;21:519. doi: 10.1097/00004694-200107000-00020. [DOI] [PubMed] [Google Scholar]
- 29.Sturm Spine. 2001;26:1389. doi: 10.1097/00007632-200106150-00025. [DOI] [PubMed] [Google Scholar]
- 30.Thompson Spine. 1995;20:1380. [PubMed] [Google Scholar]
- 31.Winter J Bone Joint Surg Am. 1968;50:1. [Google Scholar]
- 32.Winter Orthop Trans. 1983;7:32. [Google Scholar]
- 33.Winter J Bone Joint Surg Am. 1984;66:1188. [PubMed] [Google Scholar]
- 34.Winter J Pediatr Orthop. 1988;8:633. doi: 10.1097/01241398-198811000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Winter J Bone Joint Surg Am. 1996;78:300. [Google Scholar]


