Abstract
The ability of bone morphogenetic proteins (BMPs) to induce bone formation has led to an increasing interest in the potential for their use in fusion surgery. The purpose of this multi-center clinical pilot study was to evaluate the safety of one such BMP—osteogenic protein 1, in the form of OP-1 putty—combined with autograft for intertransverse process fusion of the lumbar spine in patients with symptomatic spinal stenosis and degenerative spondylolisthesis following spinal decompression. Twelve patients with spinal stenosis and degenerative lumbar spondylolisthesis underwent laminectomy and partial or complete medial facetectomy as required for decompression of the neural elements followed by intertransverse process fusion by placing iliac crest autograft and OP-1 putty between the decorticated transverse processes. No instrumentation was used. Patients were followed clinically using the Oswestry scale and radiographically using static and dynamic radiographs to assess their fusion status. Independent and blinded radiologists assessed the films for the presence of bridging bone between the transverse processes and measured translation and angulation on dynamic films using digital calipers. In addition to bridging bone, less than or equal to 5° of angular motion and less than or equal to 2 mm of translation were required to classify the patients as successfully fused, as per the definition of successful fusion provided by the FDA for use in clinical trials involving investigational devices to attain spinal fusion. Radiographic outcome was compared to a historical control (autograft alone fusion without instrumentation for the treatment of degenerative spondylolisthesis). All adverse events were recorded prospectively. The results showed 9 of the 12 patients (75%) obtained at least a 20% improvement in their preoperative Oswestry score, while 6 of 11 patients (55%) with radiographic follow-up achieved a solid fusion by the criteria used in this study. Bridging bone on the anteroposterior film was observed in 10 of the 11 patients (91%). No systemic toxicity, ectopic bone formation, recurrent stenosis or other adverse events related to the OP-1 putty implant were observed. A successful fusion was observed in slightly over half the patients in this study, using stringent criteria without adjunctive spinal instrumentation. This study did not demonstrate the superiority of OP-1 combined with autograft over an autograft alone historical control, in which the fusion rate was approximately 45%. The lack of adverse events related to the OP-1 putty implant in this study is in agreement with other studies supporting the safety of bone morphogenetic proteins in spinal surgery.
Keywords: Lumbar spine, Fusion, BMP, Spondylolisthesis, OP-1 putty
Introduction
Spinal arthrodesis is a commonly performed procedure that carries a failure rate of 10–40% [2, 4, 6, 9, 21, 22, 23, 24, 30, 45, 48, 49, 50, 51]. Many factors have been linked to fusion failure including mechanical instability, multilevel procedures, infection, poor health, smoking, and certain medications [1, 8, 10, 11, 12, 13, 15, 26, 29, 31, 42, 43, 46]. Unfortunately, failure of the fusion procedure can lead to poor clinical results. Although spinal instrumentation may decrease the rate of fusion failure, this problem has not been eliminated [23].
Since the discovery of protein factors that are able to induce bone formation by Urist in 1965, significant efforts have been made to isolate and characterize bone morphogenetic proteins (BMPs) that have the potential to stimulate bone formation and therefore enhance, augment or replace autograft bone for arthrodesis. BMPs stimulate pluripotent mesenchymal cells to differentiate into osteoblasts and produce matrix elements characteristic of a mature cell line. Osteogenic protein 1 (OP-1), also called BMP-7, is a member of the TGF-β superfamily. OP-1 strongly induces the formation of bone when implanted in soft tissues and is able to assist with fracture healing and bone fusions [16, 25, 47].
The human OP-1 gene has been cloned, allowing the purified protein product to be produced in large quantities. This recombinant product, called rhOP-1 (recombinant human OP-1), has been used in a variety of animal models, where it has been shown to produce a high rate of spinal fusion with a low risk of unwanted side effects [14, 19, 20, 27, 35, 36, 41]. Clinical studies are underway to determine the role of this growth factor in human spinal surgery. The purpose of this study was to evaluate the safety of OP-1 putty combined with autograft bone for posterolateral spinal arthrodesis in patients with symptomatic spinal stenosis in the presence of degenerative spondylolisthesis.
Materials and methods
After obtaining Human Investigations Committee approval at each of four participating centers, 12 patients with single-level, grade I or II degenerative spondylolisthesis (L3-L4 or L4-L5) and symptoms of neurogenic claudication were prospectively enrolled in this study. To meet the inclusion criteria for this study, patients had to be skeletally mature adults less than 81 years of age. All patients complained of disabling leg pain with or without back pain and had failed to improve with nonoperative treatment for at least 6 months preoperatively, and had no previous fusion attempts at the affected level. Additionally, patients who showed signs of active spinal or systemic infections, smokers, morbidly obese patients, those with a known sensitivity to collagen, women of childbearing potential and anyone who was known at the time of enrollment to require additional surgery in the next 6 months were excluded. All patients completed a detailed demographic questionnaire including an Oswestry pain scale and SF-36 form. Preoperative antero-posterior, lateral, and flexion and extension plain radiographs were obtained to evaluate the degree of translation and angulation at the level of the spondylolisthesis. Patients who had a degenerative spondylolisthesis grade III or IV, or who showed spinal instability measured on flexion/extension radiographs of greater than or equal to 20° of angular motion were excluded from this study. Advanced imaging studies were obtained (magnetic resonance imaging or myelogram/computed tomography) to confirm the presence of spinal stenosis at the level of the spondylolisthetic segment.
The study subjects (Table 1) included nine women and three men, with an average age of 68 and an age range of 45–79. Nine patients underwent surgery at the L4-L5 level while three patients underwent surgery at the L3-L4 level.
Table 1.
Demographic and clinical details of the 12 study subjects
| Age (years): mean±SD (range) | 68±8.5 (45–79) |
| Gender: n (%) | |
| Female | 9 (75%) |
| Male | 3 (25%) |
| Height (cm): mean±SD (range) | 163.8±19.8 (149.9–188.0) |
| Weight (kg): mean±SD (range) | 91.6±2.6 (55.3–111.1) |
| Level fused: n (%) | |
| L3-L4 | 3 (25%) |
| L4-L5 | 9 (75%) |
| Pre-op Oswestry score: mean±SD (range) | 41±15.6 (30.0–72.0) |
The OP-1 putty implant contained 3.5 mg lyophilized rhOP-1 mixed with 230 mg carboxymethylcellulose (CMC) and 1 g type I bone collagen. One implant was used on each side of the spine. The powdered mixture was reconstituted at the time of surgery by the addition of saline to form putty.
Surgical and postoperative protocol
After induction of anesthesia and the administration of prophylactic antibiotics, the spines were approached via a posterior midline exposure. Bilateral laminectomies with medial facetectomies were performed as necessary to decompress the neural elements. The transverse processes were then decorticated after the decompression procedure. Autograft bone was harvested from the posterior iliac crest in an amount adequate to span the space between the transverse processes. This graft was then mixed with one unit of rhOP-1 putty per side.
Postoperative course
Postoperatively, patients were fitted with a lumbosacral brace (LSO), which was worn for 3 months. Ambulation was encouraged soon after surgery, usually on postoperative day 1. Monitored physical therapy, emphasizing active exercises, was begun 6–8 weeks following surgery.
Follow-up evaluation
The patients were evaluated at 6 weeks, 3 months, 6 months, 9 months, and 12 months with radiographs and a physical exam. The Oswestry questionnaire was repeated at the 6- and 12-month time points. Clinical success was defined as at least 20% improvement in the pre-operative Oswestry score.
Two independent neuroradioiogists evaluated the radiographs using digital calipers to obtain measurements. The criteria for successful radiographic fusion were defined as the presence of bridging bone between the transverse processes at the spondylolisthetic segment, less than or equal to 5° of angulation and less than or equal to 2 mm of translation on flexion/extension radiographs. These criteria for demonstration of successful fusion were slightly more rigid than those established by the FDA as part of the required follow-up for medical devices being evaluated under FDA approved IDE studies. All radiographic criteria had to be met to be classified as a radiographic success. In the event that there was disagreement between the radiologists regarding the fusion status, a third, independent, neuroradiologist was utilized as the tiebreaker. The radiographic outcomes were compared to those reported in a historical randomized, controlled, prospective study that included a fusion arm using autograft alone without instrumentation in the treatment of symptomatic degenerative spondylolisthesis [3].
To be considered an overall success, the patient had to achieve both a 20% improvement in preoperative Oswestry score and achieve radiographic fusion.
All clinical adverse events, related or not to the use of OP-1, were recorded prospectively.
Results
Table 2 and Table 3 show the outcome of the fusion operations. Clinical success at 12 months (20% improvement in the Oswestry score) was achieved in 9 of the 12 patients (75%). Radiologic fusion at 12 months was achieved in 6 of 11 patients (55%) (Fig. 1, Fig. 2). This was similar to the fusion success rate of 45% in the autograft alone fusion group in the surgical treatment of degenerative spondylolisthesis by Fischgrund et al. [23]. One patient who was seen for clinical follow-up at 12 months did not undergo radiographic evaluation at this time point, leaving only 11 patients with evaluable radiographic data at 12 months. One patient underwent a revision posterior lumbar fusion with internal fixation for a clinical diagnosis of a pseudarthrosis with worsening back pain 8 months after the index procedure. His outcome was scored as a radiographic failure. Patients who failed to be classified as having achieved a successful fusion by radiographic standards generally demonstrated motion (above the parameters in this study) on flexion/extension radiographs. Ten of 11 patients (91%) had bridging bone between the transverse processes on anteroposterior radiographs. No patient demonstrated progression of the spondylolisthesis and one patient underwent a revision posterior lumbar fusion for a clinical diagnosis of a pseudarthrosis.
Table 2.
Radiographic and clinical outcome in the 12 study subjects
| 12-month results | Success | Failure | % Success |
|---|---|---|---|
| Clinical | 9 | 3 | 75% |
| Radiographica | 6 | 5 | 55% |
a One patient was available for their 12-month clinical assessment but did not have X-rays taken at this time
Table 3.
Translation/angulation results of the reviewers (Rev.1, Rev.2) for fusions classified as successful and those classified as failed. Ten of eleven patientsa (92%) had bridging bone at 12 months
| Translation (mm) | Angulation (degrees) | Bridging bone on A/P films: Yes/No | |
|---|---|---|---|
| Rev.1/Rev.2 | Rev.1/Rev.2 | Rev.1/Rev.2 | |
| Failed fusions | |||
| 1 | 0/1.6 | 7.2/14.2 | yes/yes |
| 2b | – | – | no/no |
| 3 | 5.2/5.8 | 12.5/10.3 | yes/yes |
| 4 | 0/0.3 | 7/5.7 | yes/yes |
| 5 | 0.6/1.5 | 7.7/8 | yes/yes |
| Successful fusions | |||
| 1 | 1.83/1.2 | 1/1 | yes/yes |
| 2 | 1.83/0 | 0.8/1.1 | yes/yes |
| 3 | 1.32/1.3 | 2.6/2.9 | yes/yes |
| 4 | 0/3.5/1.22c | 0.6/1/0c | yes/yes/yesc |
| 5 | 1.22/1.6 | 2.4/3.3 | yes/yes |
| 6 | 0/1.5 | 3.6/2.8 | yes/yes |
a The exception was patient 2, who had supplemental fixation
b No results as patient received supplemental fixation
c Third reviewer (Rev.3) used as a tie breaker
Fig. 1.
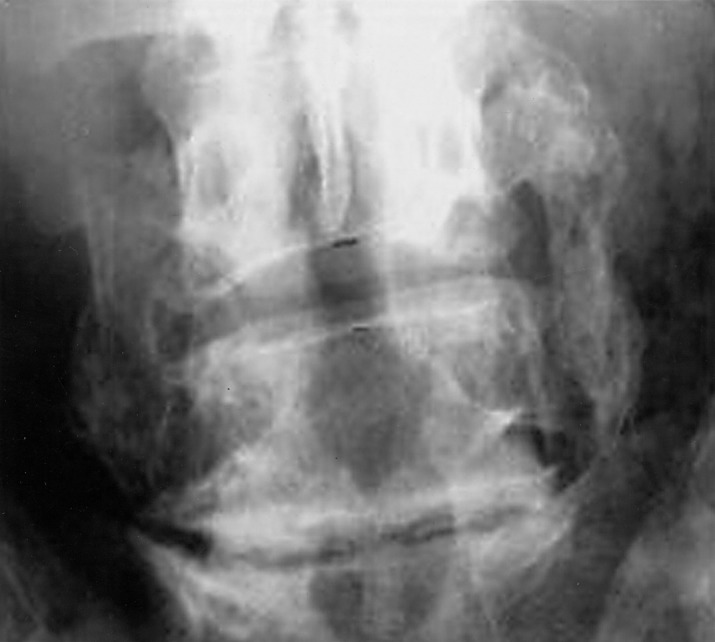
Twelve-month follow-up antero-posterior radiograph of OP-1/autograft patient showing solid bridging bone between the transverse processes
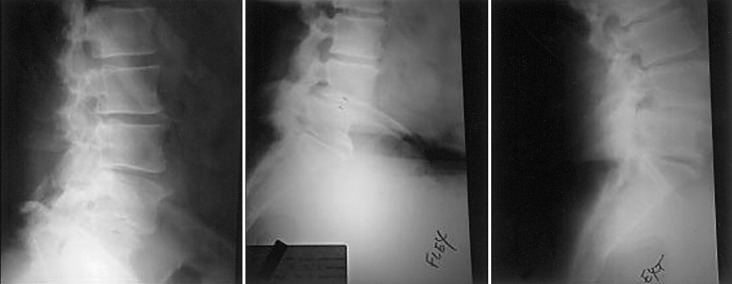
Fig. 2.
Lateral radiographs of an OP-1/autograft patient determined to be a radiographic success, in A neutral position, B flexion, and C extension
Adverse events identified among the study group included pseudarthrosis, increased back pain, donor site pain, and surgical complications, as presented in Table 4. No patient in this study had any side effects attributable to the rhOP-1 product. There were no cases of systemic toxicity, ectopic bone formation or recurrence of spinal stenosis.
Table 4.
OP-1/autograft adverse event table. There was an overall adverse event rate of 67% for the study group; only one patient required a subsequent revision procedure. None of the adverse events related to the use of OP-1 (UTI urinary tract infection)
| Patient | Event | Details |
|---|---|---|
| 2 | Pseudarthrosis | Required subsequent revision stabilization with internal fixation |
| 3 | Gastrointestinal | Nausea/vomiting |
| 4 | Increased back pain | |
| 5 | Urinary retention | |
| UTI | ||
| Iliac crest donor site pain | ||
| Left trochanteric bursitis | ||
| Broncopulmonary | Pneumonia | |
| Increased back pain | Left buttock and groin pain | |
| Trauma | Shoulder injury related to fall | |
| 6 | Cardiovascular | Pulmonary edema |
| Dermatological | IV site bruising | |
| Trauma | Back and buttock pain related to fall | |
| 7 | Dural leak/tear | |
| 8 | Trauma | Elevator injury causing back pain |
| 9 | Increased back pain | |
| 10 | Excessive epidural bleeding during decompression |
Discussion
The posterolateral region is a challenging environment in which to achieve a successful fusion following laminectomy in patients with degenerative spondylolisthesis in the absence of internal fixation. This is probably due to a combination of factors including instability and poor vascularity at the arthrodesis site as well as tensile stresses adversely affecting the fusion mass [5, 7, 36]. Clinical series confirm a poor rate of fusion in patients with degenerative spondylolisthesis, as documented by Fischgrund et al. who observed successful fusion in only 45% of patients not receiving supplemental instrumentation [23]. To improve the rate of fusion, some authors have recommended the use of supplemental instrumentation [9, 23, 39, 44], while others have argued that instrumentation may not lead to improved clinical outcomes [33, 34, 37].
The methods for judging fusion success vary widely between studies comparing the results of posterolateral fusion for degenerative spondylolisthesis, while the typical practice evaluation is based on clinical and radiographic (bridging bone) assessment. Although no method, short of surgical exploration, is considered completely accurate to determine the success of an arthrodesis, the radiographic criteria used in this study are commonly used as a noninvasive method to determine fusion success [9, 23, 30, 32, 33, 37, 44]. The use of digital calipers and independent neuroradiologists as employed in this study should increase the accuracy and objectivity compared with most other studies where less stringent fusion criteria are utilized. In addition, because instrumentation was not utilized in the current study, the ability of the radiographs to quantify ossification between the transverse processes and any residual motion is enhanced. It is known that some motion in the sagittal plane occurs in the setting of a solid posterolateral arthrodesis [3, 28, 38]; however, it is not clear how much motion should be evident to qualify as a true pseudarthrosis. This question is pertinent to the five patients in this study who demonstrated apparent bridging bone between the transverse processes on anteroposterior radiographs but demonstrated 5° or more of sagittal plane motion on flexion/extension radiographs.
Progression of the slip can occur after decompression in patients with degenerative spondylolisthesis, as has been documented by Herkowitz and Kurz [30]. In their series, 96% of patients following laminectomy alone and 28% of patients who underwent posterolateral uninstrumented fusion demonstrated progression of the slip following surgery [30]. In the present study, no patient demonstrated more than 2 mm of slip progression following posterolateral fusion, regardless of the fusion status.
The safety profile of BMPs continues to be investigated, but to date these growth factors appear to be safe. Animal studies utilizing a variety of BMPs have failed to demonstrate systemic toxicity or tumor formation in response to these substances [14, 16, 17, 18, 19, 20, 36, 40, 41]. In one study, Paramore et al. intentionally placed rhOP-1 and a carrier inside the dural sac during posterolateral fusion procedures in a canine model [40]. Although bone formed adjacent to the spinal cord causing some cord compression, no neurologic deficit, spinal cord inflammatory changes or neuronal cell death was observed. Of interest to investigators is the potential for BMPs to induce an adverse systemic antibody response. In the tibial nonunion study by Friedlaender et al. [25], low levels of anti-OP-1 antibodies developed in approximately 10% of patients treated with OP-1. All of the anti-OP-1 antibody responses were transient and all titers were low. No adverse events related to sensitization were identified, and all patients who were found to have an anti-OP-1 antibody response were healed clinically and radiographically at the 24-month follow-up. Antibody titers at this time are not available in our study population, as more advanced methods for testing for antibodies to OP-1 are currently in development.
Our study confirms an acceptable safety profile of rhOP-1 for posterolateral fusions in patients with degenerative spondylolisthesis. No patient demonstrated systemic toxicity, ectopic bone formation or implant migration into the laminectomy site.
Conclusion
The degenerative spondylolisthesis patient is a challenging model in which to obtain an arthrodesis in the absence of internal fixation. The present study evaluated the safety and efficacy of OP-1 putty in this patient population by combining OP-1 putty with autogenous bone for intertransverse process fusion. Although a successful fusion rate of only 55% was observed using stringent radiographic criteria, 91% of the patients demonstrated bridging bone, and clinical success was achieved for the 75% of patients who experienced at least a 20% improvement in their preoperative Oswestry score. No patient exhibited signs of systemic toxicity, ectopic bone formation or migration of the implant into the laminectomy site, and there were no complications related to the OP-1 putty product itself. This pilot study supports the safety of OP-1 putty when used as an adjunct to iliac crest autograft in uninstrumented posterolateral fusions. Since this study failed to demonstrate superior efficacy of the OP-1/autograft combination in comparison with autograft alone in a historical control, further study of OP-1 as a substitute for autogenous bone graft in posterolateral lumbar spinal fusion is planned. At the present time, OP-1 putty is only approved by the FDA for use in the treatment of long bone fracture nonunions and should not be used in spinal fusions until further study and subsequent clearance by the FDA is attained.
References
- 1.Albert Spine. 2000;25:123. doi: 10.1097/00007632-200001010-00021. [DOI] [PubMed] [Google Scholar]
- 2.Banwart Spine. 1995;20:1055. doi: 10.1097/00007632-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Barrick Spine. 2000;25:853. doi: 10.1097/00007632-200004010-00014. [DOI] [PubMed] [Google Scholar]
- 4.Beirne Int J Oral Maxillofac Surg. 1996;25:268. doi: 10.1016/s0901-5027(06)80053-6. [DOI] [PubMed] [Google Scholar]
- 5.Boden Orthop Clin North Am. 1998;29:603. doi: 10.1016/s0030-5898(05)70034-1. [DOI] [PubMed] [Google Scholar]
- 6.Boden Tissue Eng. 2000;6:383. doi: 10.1089/107632700418092. [DOI] [PubMed] [Google Scholar]
- 7.Boden Spine. 1995;20:102S. [PubMed] [Google Scholar]
- 8.Brantigan Spine. 1994;19:1271. doi: 10.1097/00007632-199405310-00014. [DOI] [PubMed] [Google Scholar]
- 9.Bridwell J Spinal Disord. 1993;6:461. doi: 10.1097/00002517-199306060-00001. [DOI] [PubMed] [Google Scholar]
- 10.Brown Spine. 1986;11:942. doi: 10.1097/00007632-198611000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Buttermann Spine. 1997;22:2748. doi: 10.1097/00007632-199712010-00009. [DOI] [PubMed] [Google Scholar]
- 12.Cameron Prog Clin Biol Res. 1985;187:479. [PubMed] [Google Scholar]
- 13.Carpenter J Bone Joint Surg Am. 1996;78:712. doi: 10.2106/00004623-199605000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Chirossel Stryker Spine PEEK and titanium. 2000;cages:interbody. [Google Scholar]
- 15.CohenClin Orthop 20003714610693549 [Google Scholar]
- 16.Cook Orthopedics. 1999;22:669. [PubMed] [Google Scholar]
- 17.Cook SD, Baffes GC, Wolfe MW, Sampath TK, Rueger DC (1993) Recombinant human osteogenic protein-1 (rhOP01) heals segmental long bone defects in non-human primates. Presented at the 60th Annual meeting of the American Academy of Orthopaedic Surgeons. San Francisco
- 18.Cook Spine. 1994;19:1655. doi: 10.1097/00007632-199408000-00002. [DOI] [PubMed] [Google Scholar]
- 19.CunninghamSpine 19992450910101812 [Google Scholar]
- 20.Cunningham BW, Shimanoto N, Sefter JC, et al (2000) Posterolateral spinal arthrodesis using osteogenic protein-1: an in-vivo time-course study using a canine model. NASS New Orleans
- 21.de Cleft Palate Craniofac J. 1999;36:388. doi: 10.1597/1545-1569_1999_036_0388_hibgdt_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 22.DePalma Clin Orthop. 1968;59:113. [PubMed] [Google Scholar]
- 23.Fischgrund Spine. 1997;22:2807. doi: 10.1097/00007632-199712150-00003. [DOI] [PubMed] [Google Scholar]
- 24.FranceSpine 19992455310101819 [Google Scholar]
- 25.Friedlaender J Bone Joint Surg Am. 2001;83:S151. [PMC free article] [PubMed] [Google Scholar]
- 26.Gertzbein Spine. 1998;23:2352. doi: 10.1097/00007632-199811010-00021. [DOI] [PubMed] [Google Scholar]
- 27.Grauer Spine. 2000;26:127. doi: 10.1097/00007632-200101150-00004. [DOI] [PubMed] [Google Scholar]
- 28.Greenough Spine. 1998;23:479. doi: 10.1097/00007632-199802150-00015. [DOI] [PubMed] [Google Scholar]
- 29.Heggeness Spine. 1991;16:S449. [PubMed] [Google Scholar]
- 30.Herkowitz J Bone Joint Surg Am. 1991;73:802. [PubMed] [Google Scholar]
- 31.Jacobson Radiology. 1997;204:853. doi: 10.1148/radiology.204.3.9280271. [DOI] [PubMed] [Google Scholar]
- 32.Kanayama Spine. 1998;23:767. doi: 10.1097/00007632-199804010-00004. [DOI] [PubMed] [Google Scholar]
- 33.Kimura J Spinal Disord. 2001;14:301. doi: 10.1097/00002517-200108000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Kuntz Spine. 2000;25:1132. doi: 10.1097/00007632-200005010-00015. [DOI] [PubMed] [Google Scholar]
- 35.MaginSpine 20012646911242373 [Google Scholar]
- 36.MaginSpine 20012646911242373 [Google Scholar]
- 37.McCulloch Spine. 1998;23:2243. doi: 10.1097/00007632-199810150-00020. [DOI] [PubMed] [Google Scholar]
- 38.Nachemson Spine. 1996;21:1835. doi: 10.1097/00007632-199608010-00023. [DOI] [PubMed] [Google Scholar]
- 39.Nork Spine. 1999;24:561. doi: 10.1097/00007632-199903150-00012. [DOI] [PubMed] [Google Scholar]
- 40.ParamoreNeurosurgery 199944115110232555 [Google Scholar]
- 41.Patel TC, Erulkar JS, Grauer JS (2000) OP-1 overcomes the inhibitory effects of nicotine on lumbar fusion. NASS, New Orleans [DOI] [PubMed]
- 42.Quagliano Skeletal Radiol. 1993;23:353. doi: 10.1007/BF02416992. [DOI] [PubMed] [Google Scholar]
- 43.Raiszadeh Am J Orthop. 2000;29:513. [PubMed] [Google Scholar]
- 44.Rechtine J Spinal Disord. 1996;9:382. [PubMed] [Google Scholar]
- 45.RobertsonSpine 200126147311458153 [Google Scholar]
- 46.Rothman Orthop Clin North Am. 1975;6:299. [PubMed] [Google Scholar]
- 47.Salkeld J Bone Joint Surg Am. 2001;83:803. doi: 10.2106/00004623-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 48.Schnee Spine. 1997;22:2222. doi: 10.1097/00007632-199710010-00005. [DOI] [PubMed] [Google Scholar]
- 49.Steinmann Clin Orthop. 1992;284:80. [PubMed] [Google Scholar]
- 50.Summers J Bone Joint Surg Br. 1989;71:677. doi: 10.1302/0301-620X.71B4.2768321. [DOI] [PubMed] [Google Scholar]
- 51.Younger J Orthop Trauma. 1989;3:192. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]