Abstract
Background
Risk perception and efficacy beliefs affect health behavior. The aim of this study was to measure cancer severity and curability (as proxy for risk perception and efficacy beliefs, respectively) and their association with clinical and psychosocial variables.
Methods
A consecutive sample of cancer patients were recruited and assessed for sociodemographic and medical data, patient perception of cancer severity and curability, and quality of life. The main outcome measures were the depression and anxiety components as measured by the Hospital Anxiety and Depression Scale (HADS).
Results
Subjective and objective measures of severity and curability were found to be associated. The perception of one’s own disease as severe and difficult to cure, as opposed to severe but curable, was strongly associated with depression (OR = 6.93; P = 0.048) when adjusted for potential confounding factors. Factors independently associated with anxiety were the perception of difficulty to cure (OR = 15.73; P = 0.018), having religious beliefs (OR = 49.74; P = 0.013), and metastasis (OR = 18.42; P = 0.015), when adjusted for sex, marital status, site of cancer, and time from diagnosis. Differences in curability beliefs did not affect any quality of life domain.
Conclusion
Patients and clinicians may have different perceptions of disease and treatment. The perception of control and curability must be taken into account to identify cancer patients who are suffering most and require special medical care, as these factors have an effect on depression and anxiety.
Keywords: cancer, curability, patient perception, perceived control, psychological distress
Introduction
Cancer is a serious life-threatening disease. Despite the improvements in cancer medicine that are transforming cancer into a chronic and even curable disease, patient perceptions of the disease can and do condition their experiences. Patient-perceived severity can be considered a proxy for beliefs about the objective controllability of disease, and is related to health behavior under certain conditions, such as perceived vulnerability.1 Health psychology research has demonstrated the beneficial nature of secondary control beliefs (perceived control is sustained by “primary control”, an attempt to change the environment, or “secondary control”, to adjust psychologically to one’s environment)2 and identified numerous coping strategies for individuals faced with cancer.3–5 According to the health belief model,6 perceived vulnerability and disease severity combine to form a “threat”, which in turn motivates action and is likely to change patient perceptions of control. In the extended parallel process model,7 there is a balance between threat and efficacy beliefs, ie, if efficacy beliefs exceed levels of threat, then healthy behavior is adopted (danger control), whereas if threat beliefs exceed levels of efficacy, then efforts are focused on managing fear (fear control).8 Because the potential consequences of a health threat can be diverse, it is not surprising that severity appears to be a multidimensional concept.9 Therefore, in addition to the characteristics of the illness and the efficacy of medical therapy, patient risk perception and efficacy beliefs could affect their health behavior and psychological well-being.
Evidence of an association between perceived control and adaptation to illness has been reported in women with breast cancer.10–13 Several studies have shown that perceived control decreases with age14–17 but increases with educational level.18,19 The aims of this study were to measure patient-perceived severity and curability of the disease, and to assess the relationship between patient-perceived degree of severity and curability, psychological well-being, and quality of life in patients with cancer.
Materials and methods
A sample of 135 cancer patients (24–81 years of age) participated in this cross-sectional investigation, which was approved by our local medical ethics committee. The research participants were patients affected by cancer and recruited from the Division of Oncology, Istituto Dermopatico dell’Immacolata- Istituto di Ricerca e Cura a Carattere Scientifico, a hospital located in Rome, Italy. Participants were required to be over the age of 18 years and fluent in Italian. Patients with cerebral metastasis were excluded. Subjects regularly attending the division were consecutively enrolled and asked to complete the study questionnaires during a hospital visit. Written informed consent, following thorough information provision about the purpose of the study, was obtained. One hundred and thirty-five of 137 eligible patients agreed to participate (98.5%). A structured form was used to record information concerning sociodemographic and clinical variables.
Participants were required to rate the perceived severity and curability of their disease on two ten-point scales ranging from 1 (not severe, easy to cure) to 10 (high severity, difficult to cure). Disease severity was used as a proxy for objective controllability of the disease20 and curability was used as the opportunity to exert control. Usually, disease severity is measured using a general item, and to improve its predictive utility, we weighted it according to the likelihood of disease curability.
The Functional Assessment of Cancer Therapy-General (FACT-G)21 is a 27-item scale of general questions divided into four primary quality of life domains of well-being, ie, physical, social/family, emotional, and functional. Higher scores indicate better quality of life.21,22 In this study, internal consistency was good to excellent for all the FACT-G domains, with Cronbach’s alpha coefficients ranging from 0.73 to 0.88.
The Hospital Anxiety and Depression Scale (HADS)23 is a questionnaire consisting of 14 items, ie, seven for anxiety (HADS-A) and seven for depression (HADS-D), with each being scored from 0 (not present) to 3 (highly present). Scores > 10 were chosen to identify cases of anxiety or depression.23
Data reduction and statistical analyses
For descriptive purposes, the study variables were categorized whereby subjects were subdivided into three groups with respect to years of education (<8, 8–13, >13), and time elapsed since diagnosis (<1, 1–3, >3 years). Marital status (no/yes), religious beliefs (no/yes), belonging to a religious community (no/yes), and severity and (in)curability were dichotomized into two groups (0, 1–5; 1, 6–10). Four categories based on patient perception of severity and curability of their disease were defined as follows: not severe/easy to cure; not severe/difficult to cure; severe/easy to cure; and severe/difficult to cure.
FACT-G domain and total scores were computed on a 0–100 point scale (T values) characterized by a distribution with a mean of 50 and a standard deviation of 10. The descriptive statistics included percentages or mean values, depending on the nature of each variable, as well as standard deviations, whenever applicable.
Multivariable logistic regression models were used to assess the relationship between control perception or quality of life (independent variables), and HADS-defined cases of depression or anxiety (dependent variables), adjusting for sociodemographic and medical factors. Quality of life levels were categorized as FACT-G-specific domain scores either below or above the median value of 50. The emotional well-being domain was excluded from this analysis. An additional variable considered for inclusion in the model was patient education. Medical factors considered were presence of metastasis and treatment-related side effects (no/yes). Covariates significantly associated (P < 0.10) with the outcome were entered into a multiple regression model and subjected to backward selection until all remaining covariates had a P value <0.05 adjusted for the other remaining covariates. Patient age, sex, site of cancer, and time elapsed since diagnosis were considered potentially confounding variables.
All the statistical analyses were carried out using STATA version 11.0 (Stata Corporation, College Station, TX).
Results
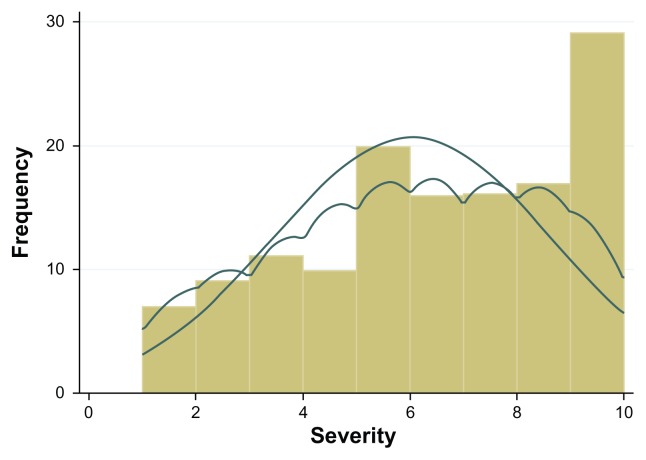
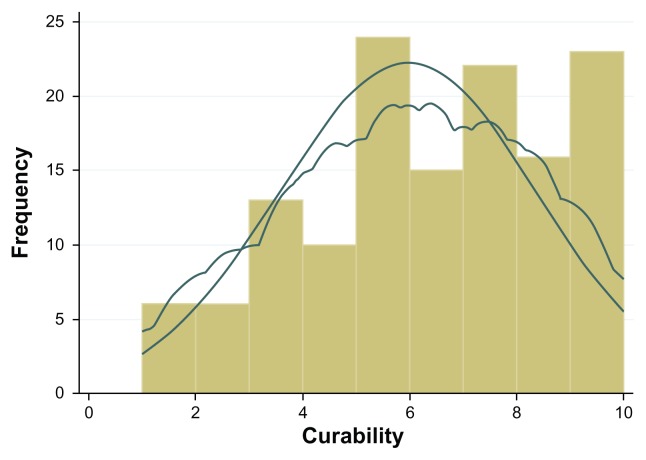
Patient sociodemographic and clinical characteristics are shown in Table 1. Close to one third of the patients (n = 40, 32.3%) scored positive for anxiety, and almost one quarter (n = 26, 21.0%) scored positive for depression. The mean patient-perceived severity score was 6.04 ± 2.60 and patient-perceived curability was 5.96 ± 2.42. Visual inspection of the distributions of raw scores revealed that both measures were negatively skewed, and probability density curves, produced by Kernel density estimation, were asymmetrical (Figures 1 and 2).
Table 1.
Sociodemographic and clinical characteristics of the study sample
| Total (N = 135) No (%) |
|
|---|---|
| Sex | |
| Males | 40 (29.6) |
| Females | 95 (70.4) |
| Age (yr)-mean (SD, median) | 58 (12, 57) |
| Education (yr) | |
| <8 49 | (36.3) |
| 8–13 | 53 (42.2) |
| >13 | 26 (19.3) |
| Marital status | |
| Never married | 33 (24.4) |
| Married | 102 (75.6) |
| Religious beliefs | 103 (76.3) |
| Belonging to a religious community | 12 (8.9) |
| Cancer type | |
| Breast | 57 (42.2) |
| Colorectal | 34 (25.2) |
| Lung | 13 (9.6) |
| Melanoma | 10 (7.4) |
| Other | 21 (15.6) |
| Length of disease (yr) | |
| <1 | 73 (54.1) |
| 1–3 | 24 (17.8) |
| >3 | 38 (28.2) |
| Treatment-related side effects | 62 (45.9) |
| Metastasis | 60 (44.4) |
Abbreviation: SD, standard deviation.
Figure 1.
Distribution of severity raw scores; histogram with normal and kernel density curves overlaid.
Figure 2.
Distribution of curability raw scores; histogram with normal and kernel density curves overlaid.
Patients who perceived their disease as severe (scores ranging from 6 to 10, n = 78, 57.8%) as opposed to not severe (scores ranging from 1 to 5, n = 57, 42.2%) were younger (55 versus 65 years; P = 0.010), had more than 8 years of education (P = 0.010), were affected by lung cancer (P = 0.043), and had metastasis (P = 0.010).
Patients who perceived their disease as difficult to cure or incurable (≤5, n = 59; 43.7%) as opposed to those who perceived their disease as curable (>5, n = 76; 56.3%) were affected by lung cancer (P = 0.017), had metastasis (P ≤ 0.001), a shorter duration of disease (5.3 versus 21.0 months, P = 0.031), and were younger (54 versus 60.5 years, P = 0.019).
Disease was judged by patients as “not severe/easy to cure” in 51 cases (37.8%), “not severe/difficult to cure” in six (4.4%), “severe/easy to cure” in 25 (18.5%), and “severe/difficult to cure” in 53 (39.8%).
As shown in Table 2, patients who assessed their disease as “severe/easy to cure” had a lower physical and emotional FACT-G well-being score compared with the other two groups, while the prevalence of HADS-defined cases of anxiety varied significantly throughout. No group differences emerged for social and functional well-being FACT-G domains or prevalence of HADS-defined cases of depression.
Table 2.
FACT domain median and range scores, and prevalence of anxiety and depression (n = 135)
| Not severe/easy to cure | Severe/easy to cure | Not severe/difficult to cure | Severe/difficult to cure | P valuea | |
|---|---|---|---|---|---|
| FACT domains | |||||
| Physical well-being | 92.8 (11–100) | 78.6 (29–100) | 83.9 (75–96) | 75.0 (25–100) | <0.001* |
| Social well-being | 75.0 ( 5–100) | 79.2 (42–96) | 79.2 (46–92) | 70.8 (10–100) | 0.634 |
| Emotional well-being | 79.2 (38–96) | 75.0 (17–92) | 77.1 (54–88) | 62.5 (4–92) | <0.001* |
| Functional well-being | 53.6 (7–93) | 42.9 (11–89) | 50.0 (43–93) | 46.4 (4–100) | 0.257 |
| Total score | 68.8 (42–90) | 66.3 (31–83) | 70.2 (63–90) | 62.5 (30–87) | 0.011 |
| HADS-Anxiety | |||||
| Yesb | 13 (27.1) | 4 (18.2) | 1 (20.0) | 22 (44.9) | |
| No | 35 (72.9) | 18 (81.8) | 4 (80.0) | 27 (55.1) | 0.053c |
| HADS-Depression | |||||
| Yesb | 9 (18.8) | 3 (13.6) | 1 (20.0) | 13 (26.5) | |
| No | 39 (81.2) | 19 (86.4) | 4 (80.0) | 36 (73.5) | 0.463c |
Notes:
Kruskal-Wallis equality of population rank test (Chi-square with ties);
group 1 differs significantly from group 4 (multiple-comparison test);
HADS subscale scores > 10;
Fisher’s Exact test.
Abbreviations: FACT, Functional Assessment of Cancer Therapy; HADS, Hospital Anxiety and Depression Scale.
Patient perception of their disease as “severe/difficult to cure” was strongly associated with HADS-defined cases of depression (odds ratio [OR] 6.93; 95% confidence interval [CI] 1.02–47.23; P = 0.048) when adjusted for sex, age, site of cancer, and time from diagnosis. Factors independently associated with HADS-defined cases of anxiety were perception of the disease being “severe/difficult to cure” (OR 15.73; P = 0.018), having religious beliefs (OR 49.74; P = 0.013), and metastasis (OR 18.42; P = 0.015), when adjusted for sex, marital status, site of cancer, and time from diagnosis.
Discussion
This study shows that health-related quality of life, as reported by an unselected hospital-based cohort of cancer patients, was generally high, except in the area of functional well-being. However, more than 20% of patients had HADS-defined depression and more than 30% had HADS-defined anxiety. In the entire sample, 58% of patients perceived their disease as severe, and 44% perceived it as difficult to cure.
Among 75 nonmetastatic patients, 71% considered their cancer to be curable; however, among 60 metastatic patients, 70% judged their disease as severe, and 62% judged it as difficult to cure. These findings suggest a marked increase in awareness of disease severity when compared with data collected for an Italian sample more than 10 years earlier.24
The main findings of this study clarify the relationship between disease curability and depression or anxiety in cancer patients. Among those patients who perceived their disease as severe and difficult to cure, there was an increased risk of HADS-defined cases of depression or anxiety, regardless of cancer site and time from diagnosis. High levels of anxiety were independently associated with metastasis and major involvement in religious activity, compared with scores for anxiety below the cut-off. Despite the cross-sectional design of our study, we can suppose that metastatic patients were more anxious than those who were nonmetastatic, and that anxious patients try to find comfort in connection with their religious traditions (illness and death raise profound spiritual questions and have a tremendous impact on patients). It is noted that this coping mechanism failed to deal with psychological distress. While some studies have shown that patients who attend religious services have better health care and mental health outcomes,25,26 not all do so.27–29 Recently, we have found that the absence of anxiety disorder, and coping strategies characterized by acceptance and positive reinterpretation of the stressor, independently increased the likelihood of existential well-being (OR 4.5 and OR 7.7, respectively), whereas religious well-being was not significantly associated with these variables.30
The diagnosis of cancer is generally regarded as a low-control situation. In our sample, more severe cancer was not considered a threatening situation by all patients, and it is the perception of curability (easy or difficult) that determines controllability. In a recent prospective study,31 a decline in perception of control was noted both before and after diagnosis, which resulted in perception being maladaptive because it accounted for psychological distress at 6 and 12 months after diagnosis, regardless of the prognosis. The authors assumed that changes in control perception could be the result of the diagnosis itself, and that they can be caused by being confronted with an event over which no control could be exerted (ie, a diagnosis of cancer), by the limited possibility to exert control over the consequences of the event (ie, treatment), or by a lack of opportunity to control the course of the disease. In our sample, we noted tremendous variability in time elapsed since diagnosis, making it difficult to analyze the impact of diagnosis and treatment.
It should be noted that this study has some limitations. Although we used standardized validated questionnaires to measure quality of life and psychological distress, limitations inherent in patient self-assessment and choice of cut-off points should be considered. A clinical diagnosis of mental disease was not available. A cross-sectional study cannot generally identify the direction of detected associations, and we cannot state with certainty that the low-control disease perception (severe and difficult to cure) is a cause rather than a consequence of psychological distress. Longitudinal prospective studies are needed to measure the incidence of psychological distress and its time of occurrence.
The findings of this study have implications for both screening policies and practical management of patients with cancer. Our sample is characterized by high quality of life scores. However, of great concern is the high prevalence of HADS-defined depression and anxiety in patients with a perception of low-control disease. Clinician support can reduce external sources of anxiety, but not internal ones. The assistance of trained counselors is needed for patients to learn how to manage internal fear and uncertainty (a common emotional response to health threats) and to improve or gain a sense of control even in situations where there are few opportunities to do so.32,33 Psychological interventions emphasizing a sense of control over illness could be effective in enhancing well-being.
Conclusion
The use of validated screening questionnaires such as HADS, facilitating recognition of psychological problems in the nonpsychiatric setting, and use of easy tools such as ten-point scales to measure severity and curability are useful in identifying individuals who may benefit from professional assessment and treatment, and should become part of the routine management of cancer patients. As noted by Bárez et al,11 the evolution of distress can be predicted from the initial value and rate of change of control perception.
Acknowledgment
We would like to thank all the patients who participated in this study.
Footnotes
Disclosure
The authors indicate no potential conflicts of interest in this work.
References
- 1.Albarracin D, Gillette JC, Earl AN, Glasman LR, Durantini MR, Ho MH. A test of major assumptions about behavior change: a comprehensive look at the effects of passive and active HIV-prevention interventions since the beginning of the epidemic. Psychol Bull. 2005;131:856–897. doi: 10.1037/0033-2909.131.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothbaum F, Weisz JR, Snyder SS. Changing the world and changing the self: a two process model of perceived control. J Pers Soc Psychol. 1982;42:5–37. [Google Scholar]
- 3.Carver CS, Pozo C, Harris SD, et al. How coping mediates the effect of optimism on distress: a study of women with early stage breast cancer. J Pers Soc Psychol. 1993;65:375–390. doi: 10.1037//0022-3514.65.2.375. [DOI] [PubMed] [Google Scholar]
- 4.Thompson SC, Sobolew-Shubin A, Galbraith ME, Schwankovaky L, Cruzen D. Maintaining perceptions of control: finding perceived control in low-control circumstances. J Pers Soc Psychol. 1993;64:293–304. doi: 10.1037//0022-3514.64.2.293. [DOI] [PubMed] [Google Scholar]
- 5.Weisz JR, McCabe MA, Dennig MD. Primary and secondary control among children undergoing medical procedures: Adjustment as a function of coping style. J Consult Clin Psychol. 1994;62:324–332. doi: 10.1037//0022-006x.62.2.324. [DOI] [PubMed] [Google Scholar]
- 6.Hochbaum GM. Public Participation in Medical Screening Programs: A Sociopsychological Study. Washington, DC: US Government Printing Office; 1958. [Google Scholar]
- 7.Witte K. Putting the fear back into fear appeals: the extended parallel process model. Commun Monogr. 1992;59:329–349. [Google Scholar]
- 8.Witte K. Fear as motivator, fear as inhibitor: using the extended parallel process model to explain fear appeal successes and failures. In: Andersen PA, Guerrero LK, editors. Handbook of Communication and Emotion: Research, Theory, Applications and Contexts. San Diego, CA: Academic Press; 1998. [Google Scholar]
- 9.Milne S, Sheeran P, Orbell S. Prediction and intervention in healthrelated behavior: a meta-analytic review of protection motivation theory. J Appl Soc Psychol. 2000;30:106–143. [Google Scholar]
- 10.Bárez M, Blasco T, Fernández-Castro J, Viladrich C. A structural model of the relationships between perceived control and adaptation to illness in women with breast cancer. J Psychosoc Oncol. 2007;25:21–43. doi: 10.1300/J077v25n01_02. [DOI] [PubMed] [Google Scholar]
- 11.Bárez M, Blasco T, Fernández-Castro J, Viladrich C. Perceived control and psychological distress in women with breast cancer: a longitudinal study. J Behav Med. 2009;32:187–196. doi: 10.1007/s10865-008-9180-5. [DOI] [PubMed] [Google Scholar]
- 12.Henselmans I, Sanderman R, Baas PC, Smink A, Ranchor AV. Personal control after a breast cancer diagnosis: stability and adaptive value. Psychooncology. 2009;18:104–108. doi: 10.1002/pon.1333. [DOI] [PubMed] [Google Scholar]
- 13.Henselmans I, Sanderman R, Helgeson VS, de Vries J, Smink A, Ranchor AV. Personal control over the cure of breast cancer: adaptiveness, underlying beliefs and correlates. Psychooncology. 2010;19:525–534. doi: 10.1002/pon.1599. [DOI] [PubMed] [Google Scholar]
- 14.Bailis DS, Chipperfield JG. Compensating for losses in perceived personal control over health: a role for collective self-esteem in healthy aging. J Gerontol B Psychol Sci Soc Sci. 2002;57:531–539. doi: 10.1093/geronb/57.6.p531. [DOI] [PubMed] [Google Scholar]
- 15.Chipperfield JG, Campbell DW, Perry RP. Stability in perceived control: implications for health among very old community-dwelling adults. J Aging Health. 2004;16:116–147. doi: 10.1177/0898264303260447. [DOI] [PubMed] [Google Scholar]
- 16.Kempen GIJM, Ranchor AV, Ormel J, Van Sonderen E, Van Jaarsveld CHM, Sanderman R. Perceived control and long-term changes in disability in late middle-aged and older persons: an eight-year follow-up study. Psychol Health. 2005;20:193–206. [Google Scholar]
- 17.Ruthig JC, Chipperfield JG, Bailis DS, Perry RP. Perceived control and risk characteristics as predictors of older adults’ health risk estimates. J Soc Psychol. 2008;148:667–688. doi: 10.3200/SOCP.148.6.667-688. [DOI] [PubMed] [Google Scholar]
- 18.Bailis DS, Segall A, Mahon MJ, Chipperfield JG, Dunn EM. Perceived control in relation to socioeconomic and behavioral resources for health. Soc Sci Med. 2001;52:1661–1676. doi: 10.1016/s0277-9536(00)00280-x. [DOI] [PubMed] [Google Scholar]
- 19.Mirowsky J, Ross CE. Education, personal control, lifestyle and health. Res Aging. 1998;20:415–449. [Google Scholar]
- 20.Chrinstensen AJ, Turner CW, Smith TW. Health locus of control and depression in end-stage renal disease. J Consult Clin Psychol. 1991;59:419–424. doi: 10.1037//0022-006x.59.3.419. [DOI] [PubMed] [Google Scholar]
- 21.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy (FACT) scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 22.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system properties, applications and interpretation. Health Qual Life Outcomes. 2003;16:1–79. doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An update literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 24.The Italian Group for the Evaluation of Outcomes in Oncology (IGEO). Awareness of disease among Italian cancer patients. Is there a need for further improvement in patient information? Ann Oncol. 1999;10:1095–1100. [No authors listed] [PubMed] [Google Scholar]
- 25.Gillum RF, King DE, Obisean TO, Koenig HG. Frequency of attendance at religious services and mortality in a US national cohort. Ann Epidemiol. 2008;18:124–129. doi: 10.1016/j.annepidem.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koenig HG. Religion and remission of depression in medical inpatients with heart failure/pulmonary disease. J Nerv Ment Dis. 2007;195:389–395. doi: 10.1097/NMD.0b013e31802f58e3. [DOI] [PubMed] [Google Scholar]
- 27.Burker EJ, Evon DM, Sedway JA, Egan T. Religious and non-religious coping in lung transplant candidates: does adding God to the picture tell us more? J Behav Med. 2005;28:513–526. doi: 10.1007/s10865-005-9025-4. [DOI] [PubMed] [Google Scholar]
- 28.Fitchett G, Murphy PE, Kim J, Gibbons JL, Cameron JR, Davis JA. Religious struggle: prevalence, correlates and mental health risks in diabetic, congestive heart failure, and oncology patients. Int J Psychiatry Med. 2004;34:179–196. doi: 10.2190/UCJ9-DP4M-9C0X-835M. [DOI] [PubMed] [Google Scholar]
- 29.Pargament KI, Koenig HG, Tarakeshwar N, Hahn J. Religious struggle as a predictor of mortality among medically ill elderly patients: a 2-year longitudinal study. Arch Intern Med. 2001;161:1881–1885. doi: 10.1001/archinte.161.15.1881. [DOI] [PubMed] [Google Scholar]
- 30.Mazzotti E, Mazzuca F, Sebastiani C, Scoppola A, Marchetti P. Predictors of existential and religious well-being among cancer patients. Support Care Cancer. 2011;19:1931–1937. doi: 10.1007/s00520-010-1033-4. [DOI] [PubMed] [Google Scholar]
- 31.Ranchor AV, Wardle J, Steptoe A, Henselmans I, Ormel J, Sanderman R. The adaptive role of perceived control before and after cancer diagnosis: a prospective study. Soc Sci Med. 2010;70:1825–1831. doi: 10.1016/j.socscimed.2009.10.069. [DOI] [PubMed] [Google Scholar]
- 32.Carter S. Anxiety in high-risk or already diagnosed cancer patients: recognizing and treating external and internal sources. Community Oncol. 2008;5:C113. [Google Scholar]
- 33.Thunè-Boyle ICV, Myers LB, Newman SP. The role of illness beliefs, treatment beliefs, and perceived severity of symptoms in explaining distress in cancer patients during chemotherapy treatment. Behav Med. 2006;32:19–29. doi: 10.3200/BMED.32.1.19-29. [DOI] [PubMed] [Google Scholar]