Abstract
Patients suffering from neurogenic intermittent claudication secondary to lumbar spinal stenosis have historically been limited to a choice between a decompressive laminectomy with or without fusion or a regimen of non-operative therapies. The X STOP Interspinous Process Distraction System (St. Francis Medical Technologies, Concord, Calif.), a new interspinous implant for patients whose symptoms are exacerbated in extension and relieved in flexion, has been available in Europe since June 2002. This study reports the results from a prospective, randomized trial of the X STOP conducted at nine centers in the U.S. Two hundred patients were enrolled in the study and 191 were treated; 100 received the X STOP and 91 received non-operative therapy (NON OP) as a control. The Zurich Claudication Questionnaire (ZCQ) was the primary outcomes measurement. Validated for lumbar spinal stenosis patients, the ZCQ measures physical function, symptom severity, and patient satisfaction. Patients completed the ZCQ upon enrollment and at follow-up periods of 6 weeks, 6 months, and 1 year. Using the ZCQ criteria, at 6 weeks the success rate was 52% for X STOP patients and 10% for NON OP patients. At 6 months, the success rates were 52 and 9%, respectively, and at 1 year, 59 and 12%. The results of this prospective study indicate that the X STOP offers a significant improvement over non-operative therapies at 1 year with a success rate comparable to published reports for decompressive laminectomy, but with considerably lower morbidity.
Keywords: Lumbar spinal stenosis, Surgical treatment, Conservative treatment, Randomized study, Prospective study
Introduction
Neurogenic intermittent claudication secondary to lumbar spinal stenosis (LSS) was first described by Verbiest [59]. The definition, etiology, clinical symptoms, incidence, and treatment of LSS have since been well documented [1, 3, 5, 11, 13, 15, 21, 22, 23, 24, 25, 26, 38, 42, 43, 44, 59, 60, 61] and are generally attributed to neural compression at one or more motion segments.
Herniated nucleus pulposus (HNP) can cause central stenosis, defined as neural compression of the spinal cord and/or cauda equina [13, 32, 59, 65]. Thickened laminae, hypertrophied ligamentum flava, spondylolisthesis, disc bulge, and facet arthrosis can lead to central, lateral recess, or foraminal stenosis [1, 13, 14, 17, 24, 60].
The LSS is often a position-dependent condition where symptoms such as leg pain, tingling, numbness, and weakness are exacerbated in lumbar extension and relieved in standing flexion or sitting [5, 7, 10, 23, 37, 38, 43, 59, 62]. The primary level affected is L4–L5, followed by L3–L4, L5–S1, L2–L3, and L1–L2 [1, 24, 25, 50, 59]. Patients with stable symptoms are generally treated with a regimen of non-operative treatments that may include epidural steroids, calcitonin, and physical therapy. For patients who fail to respond, surgery may be considered. Depending on the anatomic region of the stenosis, decompressive surgical options include laminectomy, laminotomy, or foraminotomy. In addition, fusion may be indicated in cases where the motion segment is believed to be unstable. The success rate of decompressive surgery varies greatly due to a number of factors such as patient selection, surgical technique, and outcomes measure. A meta-analysis of 74 studies of surgery for lumbar spinal stenosis reported a mean rate of good to excellent outcomes of 64% in the first year [58].
A novel alternative therapy to conservative treatment and decompressive surgery has been developed for patients suffering from LSS [66, 67, 68, 69]. The X STOP Interspinous Process Distraction (IPD) System (X STOP) is indicated in patients whose symptoms are exacerbated in extension and relieved in flexion. Implanted between the spinous processes, the X STOP reduces extension at the symptomatic level(s), yet allows flexion and unrestricted axial rotation and lateral bending [33, 63, 64].
This study compares the 1-year clinical results of the X STOP and non-operative therapy (NON OP) in treating patients with LSS.
Materials and methods
Patient selection
Nine centers randomized 200 patients between May of 2000 and July of 2001, in a prospective, controlled trial. The inclusion criteria for enrollment eligibility specified that patients be 50 years of age or older with leg, buttock, or groin pain, with or without back pain, that could be relieved during flexion. Eligible patients had to be able to sit for 50 minutes without pain, walk 50 feet or more, and have completed at least 6 months of non-operative therapy. Stenosis was confirmed by CT or MRI scans at one or two levels. Finally, eligible patients had to be capable of complying with scheduled clinical and radiographic follow-up evaluations. Primary exclusion criteria included a fixed motor deficit, cauda equina syndrome, significant lumbar instability, previous lumbar surgery, significant peripheral neuropathy or acute denervation secondary to radiculopathy, scoliotic Cobb angle greater than 25°, spondylolisthesis greater than grade 1.0 (on a scale of 1–4) at the affected level, sustained pathologic fractures, or severe osteoporosis of the vertebrae and/or hips, obesity, active infection or systemic disease, Paget’s disease or metastasis to the vertebrae, or steroid use for more than 1 month within 12 months preceding the study.
Upon enrollment, patients were randomized to either the X STOP group or the NON OP group using block randomization by surgical center. An individual not involved in the treatment or care of the patients performed the randomization and informed the surgeon of its result. Patients randomized to the NON OP group received at least one epidural steroid injection and could receive, nonsteroidal anti-inflammatories (NSAIDs), analgesics, and physical therapy. Physical therapy consisted of back school and modalities such as ice packs, heat packs, massage, stabilization exercises, and pool therapy. Braces, such as abdominal binders and corsets, were permitted, but body jackets and chair-back braces were not.
Outcomes assessment
Data were collected prior to the initial treatment, and at 6 weeks, 6 months, and 1 year following the initial treatment using the Medical Outcomes Study Short Form-36 (SF-36) and a validated outcomes measurement specific to lumbar spinal stenosis, the Zurich Claudication Questionnaire (ZCQ) [53, 54], also known as the Swiss Spinal Stenosis Questionnaire (SSS) [40] and the Zurich Claudication Score (ZCS) [9].
The ZCQ captures data in three distinct domains: symptom severity; physical function; and post-treatment patient satisfaction. The symptom severity domain consists of seven questions, each of which can receive a score from 1 to 5. The physical function domain contains five questions, each of which can receive a score from 1 to 4. The scores from each domain are then averaged. An average score of 1 is the best possible outcome and represents no pain in the symptom severity domain and no limitation in function in the physical function domain. Patient satisfaction is measured by averaging the scores of six questions, each of which can be answered as “very satisfied” (a score of 1) “somewhat satisfied” (a score of 2) “somewhat dissatisfied” (a score of 3), and “very dissatisfied” (a score of 4). Treatment is considered successful if the patient is at least “somewhat satisfied” (an average score of 2.5) and has at least a 0.5 improvement in both symptom severity and physical function [53, 54].
At each follow-up visit, patients were asked to complete the SF-36 and ZCQ. The mean pre-treatment and post-treatment SF-36 questionnaire results for each domain were compared between the X STOP and NON OP groups at each time point using an ANOVA test (p<0.05). Success rates based on the ZCQ criteria compared the X STOP and NON OP groups at each time point using the Fisher exact test (p<0.05).
Operative technique
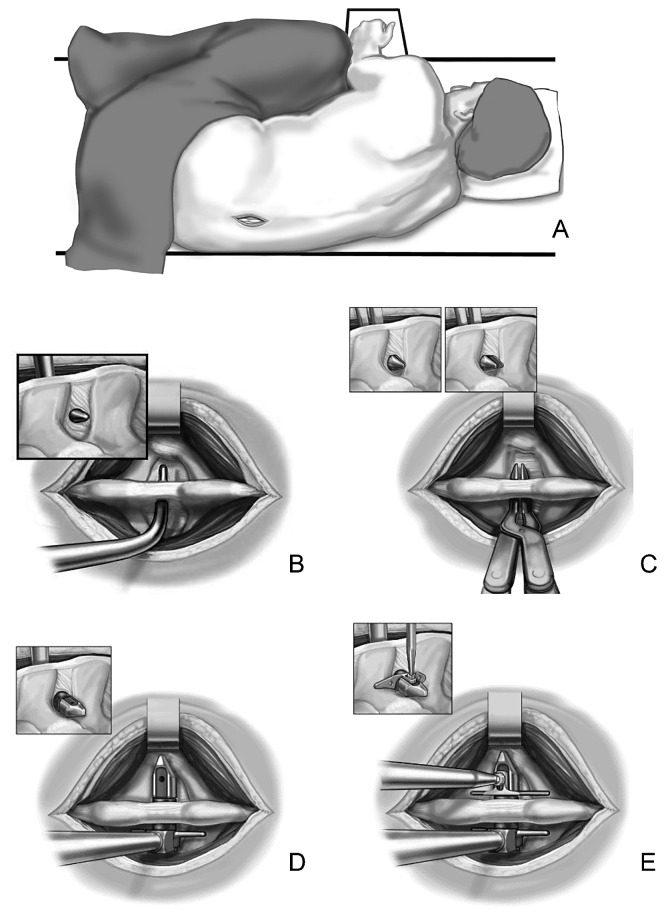
Patients enrolled in the X STOP group underwent surgery for implantation of the interspinous implant. Patients were placed on a radiolucent table in the right lateral decubitus position and asked to flex their spine (Fig. 1A). After the operative level(s) were confirmed through fluoroscopy, patients received a local anesthetic. General anesthesia was not typically required. A mid-sagittal incision of approximately 4 cm was made over the spinous processes of the stenotic level(s) and the musculature was elevated to the level of laminae and facets. Occasionally, hypertrophied facets that blocked entry to the anterior interspinous space were partially trimmed to enable anterior placement of the implant. A curved dilator was inserted in the anterior margin of the interspinous space to pierce the interspinous ligament (Fig. 1B). A sizing distractor was then inserted to determine the appropriate implant size (Fig. 1C). The X STOP (Fig. 2) was then secured to the insertion instrument and inserted into the interspinous space (Fig. 1D). An attempt was made to place the implant as close to the posterior aspect of the lamina as possible. An adjustable wing was fastened to the implant and positioned as close to the midline as possible (Figs. 1E, 3). The incision was closed. Patients without significant comorbidities were typically allowed to return home on the same day as surgery.
Fig. 1.
A The patient in the right lateral decubitus position with an incision at the stenotic level. B The curved dilator inserted in the anterior margin of the interspinous space. C A sizing distractor inserted to determine the implant size. D The X STOP inserted into the interspinous space. E The adjustable wing fastened to the implant
Fig. 2.
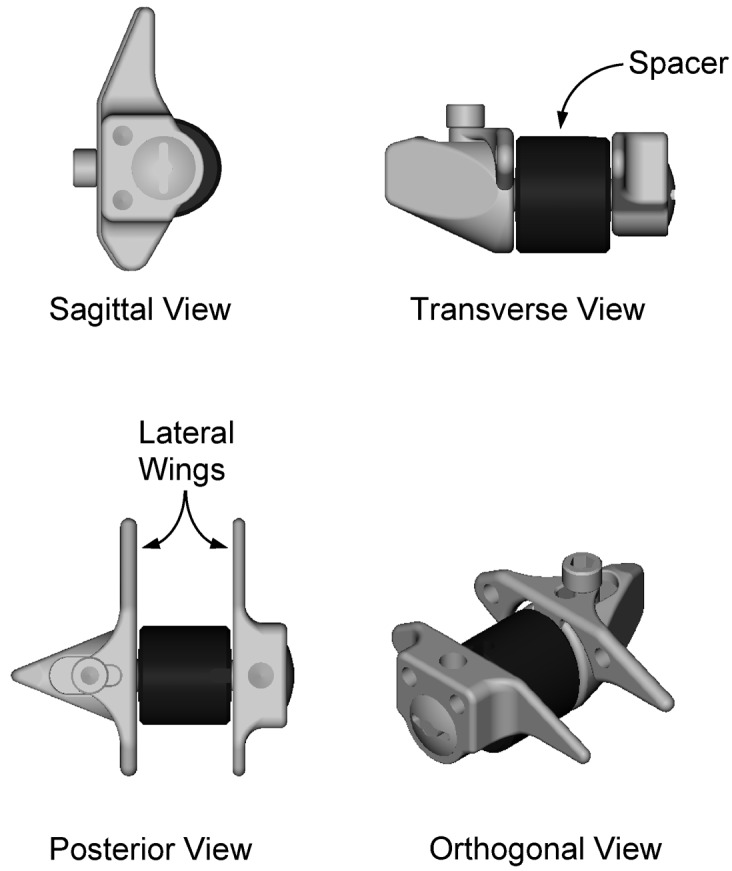
A sagittal, transverse, posterior, and orthogonal view of the X STOP implant. The spacer is produced in 6-, 8-, 10-, 12-, and 14-mm sizes to accommodate a range of interspinous heights. The lateral wings prevent lateral and anterior implant migration
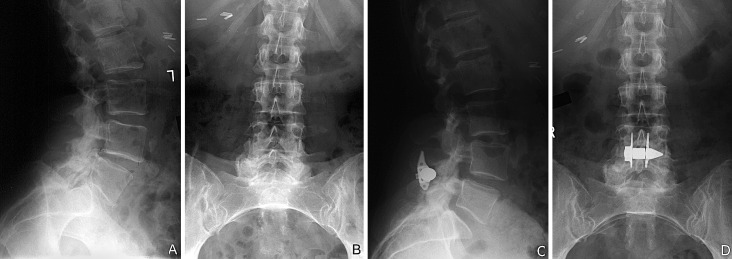
Fig. 3 A.
Pre-operative lateral and B anteroposterior (AP) radiographs. C Post-operative lateral and D AP radiographs of the same patient
Results
Demographics
Of 200 patients enrolled in this study, 191 patients were treated: 100 in the X STOP group and 91 in the NON OP group. Most of the 9 NON OP patients who withdrew from the study before receiving their initial epidural injection entered the study hoping to be randomized to the X STOP group. When they were informed of the randomization results, they withdrew from the study almost immediately. There were no statistical differences between the mean age, height, and weight for the two groups (Table 1). Sixty-four percent of the X STOP patients and 48% of the NON OP patients received at least one epidural injection before entering the study. At each follow-up period patients in both groups were lost to follow-up due to missed office visits, additional surgery, study withdrawal, and death. At each time point, more patients in the NON OP group were lost to follow-up than in the X STOP group (Table 2). Patients who (a) had the implant removed, (b) formally withdrew from the study, or (c) went on to a laminectomy were considered failures from the time of the event until the end of the study. Patients who missed appointments, but were still officially in the study, were not considered failures unless their ZCQ scores dictated so. The majority of patients were treated for single-level stenosis that occurred primarily at L4–L5 (Table 3).
Table 1.
Patient demographics
| X STOP | NON OP | |
|---|---|---|
| Age (years) | 69.9 | 68.6 |
| Male/female (%) | 57/43 | 52/48 |
| Height (cm) | 171 | 169 |
| Weight (kg) | 80.4 | 81.4 |
| Pain duration (years) | 3.5 | 4.7 |
| Pre-treatment epidural (%) | 64 | 48 |
| Employed (%) | 32 | 31 |
| Workers compensation (%) | 3 | 2 |
Table 2.
Reasons for loss to follow-up. ZCQ Zurich Claudication Questionnaire
| X STOP | NON OP | |||||
|---|---|---|---|---|---|---|
| 6 weeks | 6 months | 1 year | 6 week | 6 months | 1 year | |
| Completed ZCQ | 94 | 88 | 88 | 72 | 63 | 68 |
| Missed office visit | 4 | 6 | 2 | 16 | 11 | 1 |
| Death | 1 | 2 | 2 | 0 | 1 | 2 |
| Implant removed | 1 | 1 | 2 | 0 | 0 | 0 |
| Withdrew | 0 | 1 | 3 | 9 | 11 | 12 |
| Laminectomy | 0 | 2 | 3 | 3 | 14 | 17 |
Table 3.
Number of levels treated and treated level
| X STOP (%) | NON OP (%) | |
|---|---|---|
| No. of levels | ||
| 1 | 76 | 80 |
| 2 | 24 | 20 |
| Treated level | ||
| L2–L3 | 4 | 1 |
| L3–L4 | 30 | 30 |
| L4–L5 | 89 | 83 |
| L5–L6 | 1 | 2 |
ZCQ
Pre-treatment enrollment scores in the symptom severity and physical function domains were not significantly different between the patients in the X STOP and NON OP groups (Table 4). At each follow-up time point, the X STOP group had a significantly greater percentage of patients who improved in the symptom severity domain than did the NON OP group (Fig. 4). The improvement in the physical function domain was significantly greater for the X STOP group than for NON OP group at all follow-up time points (Fig. 5), as was the proportion of X STOP patients who were satisfied with their treatment compared with NON OP patients (Fig. 6). Combining all three domains, the overall success rate of the X STOP treatment was significantly greater than the NON OP treatment rate at all time points (Fig. 7).
Table 4.
Pre-treatment ZCQ scores for the symptom severity (SS) and physical function (PF) domains. Mean with range in parentheses
| X STOP | NON OP | |
|---|---|---|
| SS | 3.14 (2.00–4.43) | 3.12 (2.00–4.29) |
| PF | 2.48 (1.60–3.60) | 2.49 (1.40–3.80) |
Fig. 4.
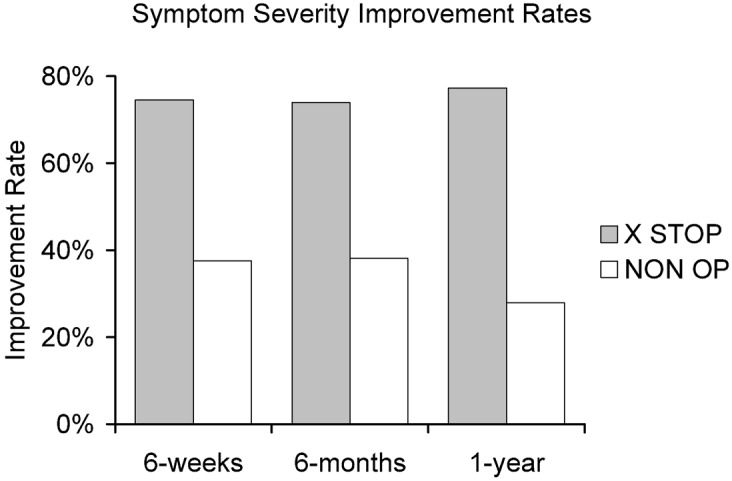
Improvement in the symptom severity of X STOP and NON OP patients at 6 weeks, 6 months, and 1 year. The X STOP patients are significantly more pain free at each time point
Fig. 5.
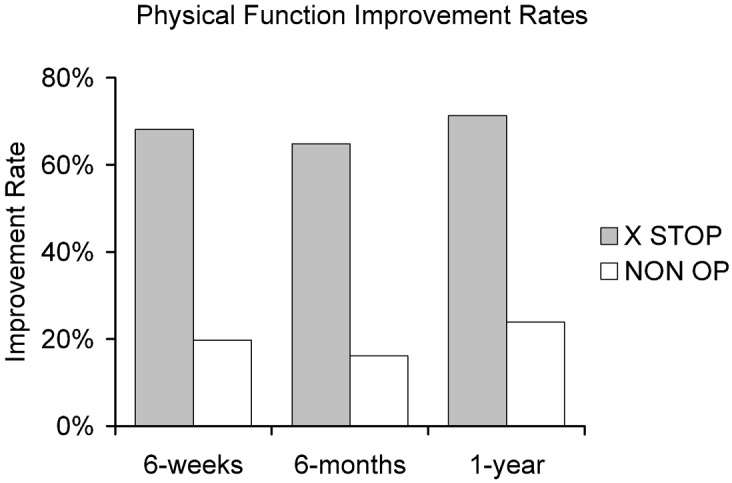
Improvement in the physical function of X STOP and NON OP patients at 6 weeks, 6 months, and 1 year. The X STOP patients have significantly improved function at each time point
Fig. 6.
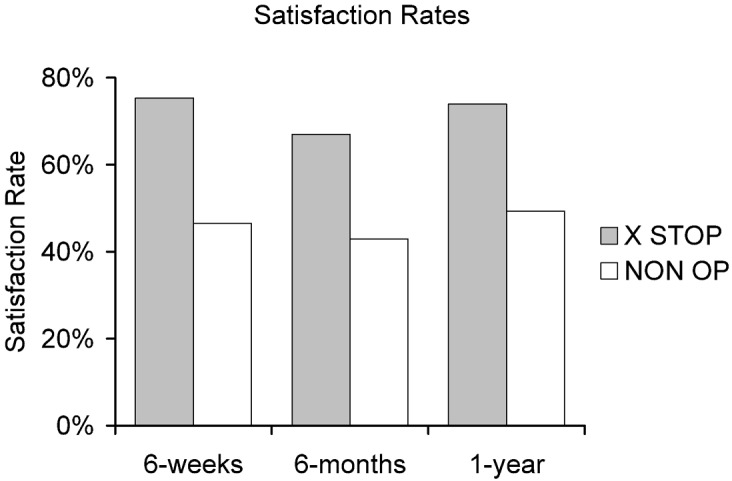
Satisfaction rates of the X STOP and NON OP patients at each time point. The X STOP patients are significantly more satisfied than the NON OP patients with their treatment
Fig. 7.
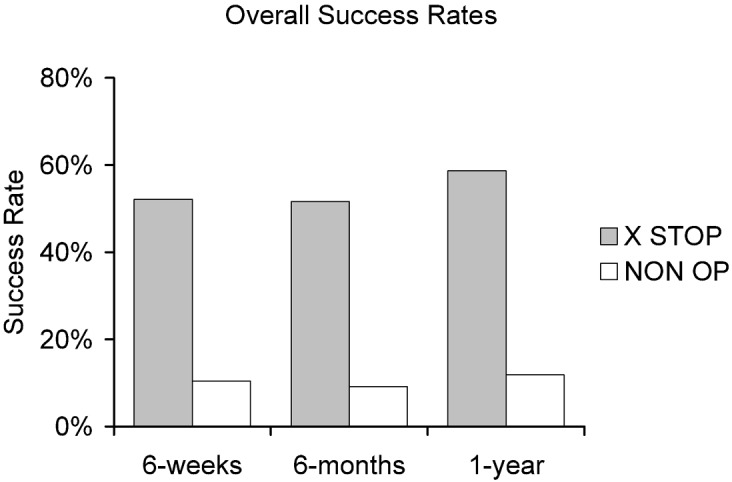
In order to be considered a clinical success, a patient must be significantly improved in the symptom severity and physical function domains, and be satisfied with the treatment outcome. Significantly more X STOP patients are a clinical success than NON OP patients
SF-36
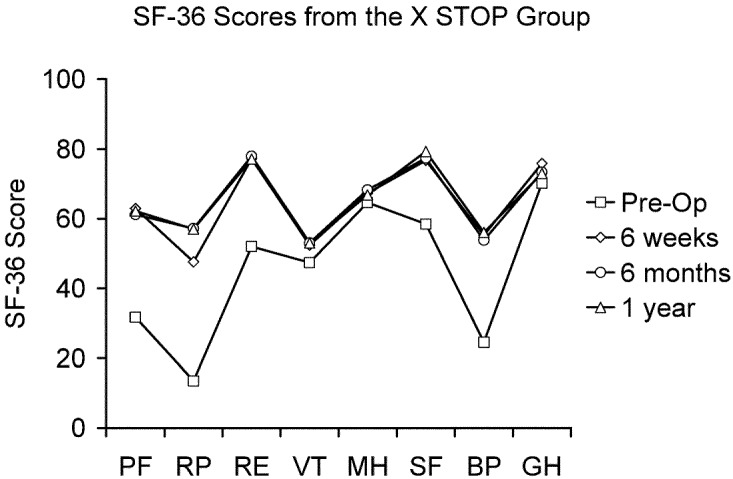
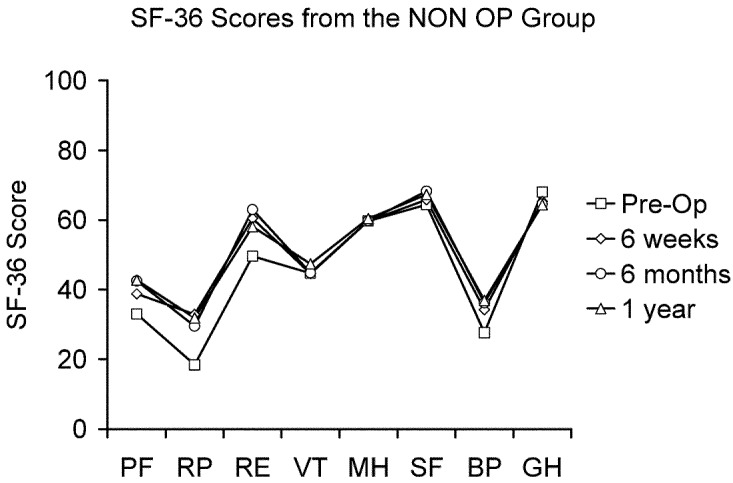
There were no significant differences in the pre-treatment enrollment scores between the X STOP and NON OP groups for any SF-36 domain (Table 5). At the 6-week, 6-month, and 1-year post-treatment follow-up time points, the X STOP group scored significantly better than the NON OP group in every domain. In addition, at each time point, the mean scores in each category for the X STOP group were significantly better than the respective pre-treatment scores (Fig. 8), whereas in the NON OP group, none of the mean scores was significantly better (Fig. 9).
Table 5.
SF-36 scores
| SF-36 domain | Current study | Hozack, 97 [19] | Strömqvist, 01 [52] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X STOP | NON OP | Disc herniation | Central stenosis | Lateral stenosis | ||||||
| Pre-op | 1-year post-op | Pre-treatment | 1-year post-treatment | Pre-op | 2-year post-op | Pre-op | 1-year post-op | Pre-op | 1-year post-op | |
| Physical function | 31.7 | 62.2 | 33.0 | 42.7 | 39.7 | 72 | 24 | 43 | 30 | 50 |
| Role physical | 13.5 | 57.0 | 17.3 | 31.9 | 10.9 | 64 | 8 | 25 | 8 | 35 |
| Bodily pain | 24.5 | 56.1 | 28.2 | 36.9 | 24 | 67.8 | 25 | 45 | 25 | 45 |
| General health | 70.2 | 73.0 | 69.9 | 64.4 | 67.6 | 73.5 | 47 | 49 | 47 | 52 |
| Vitality | 47.4 | 53.0 | 16.9 | 47.4 | 42.4 | 61.2 | 32 | 44 | 30 | 50 |
| Social function | 58.5 | 79.3 | 63.9 | 67.3 | 42.2 | 82.9 | 45 | 62 | 48 | 62 |
| Role emotional | 52.0 | 77.1 | 49.0 | 58.1 | 57 | 86.2 | 25 | 44 | 28 | 50 |
| Mental health | 64.6 | 66.8 | 61.2 | 60.4 | 58.9 | 77.3 | 60 | 67 | 55 | 67 |
Fig. 8.
The pre-op, 6-week, 6-month, and 1-year SF-36 scores for the X STOP group. The scores at all follow-up time points are significantly improved over the pre-op scores. PF physical function, RP role physical, RE role emotional, VT vitality, MH mental health, SF social function, BP bodily pain, GH general health
Fig. 9.
The pre-treatment, 6 week, 6 month, and 1 year SF-36 scores for the NON OP group. The scores at all follow-up time points are not significantly different from the pre-treatment scores. PF physical function, RP role physical, RE role emotional, VT vitality, MH mental health, SF social function, BP bodily pain, GH general health
Discussion
It is well understood that flexion relieves the symptoms of LSS and extension aggravates the symptoms [1, 5, 8, 11, 12, 16, 24, 29, 34, 36, 49, 57, 59]. In addition, radiographic, histologic, and biomechanical studies have demonstrated that the pain relief is a result of an increase in the dimensions of the canal and foramen [7, 14, 23, 37, 43, 62]. Based on these clinical observations, the X STOP was conceived as a mechanical means of providing effective pain relief for LSS patients by placing the stenotic level(s) in slight flexion and restricting extension.
Biomechanical findings
In vitro biomechanical testing demonstrated that by distracting the interspinous processes the X STOP increased the dimensions of the spinal canal and neural foramina at the implanted level in extension, but it did not alter the dimensions of the adjacent, intact levels in the extended, flexed, or neutral positions [41]. Also, the X STOP significantly reduced the pressure in the posterior annulus and nucleus at the implanted disc space in extension and neutral, without altering the mean pressures at adjacent levels [55, 56].
The results of the current study suggest that these biomechanical effects produce a clinical benefit for LSS patients treated with the X STOP. Approximately 75% of X STOP patients experienced clinical improvements in symptom severity at the 6-week, 6-month, and 1-year follow-up periods, and a similar number had clinically improved physical function at all time points. Nearly 70% of the patients were satisfied with the results of their treatment at all time points.
Rigor of the ZCQ
Previous studies using different outcomes measurements other than the ZCQ report substantially higher improvement rates for non-operative therapy patients than found in the current study. Amundsen et al. reported on 100 patients assigned to a surgical or conservative treatment group based on their symptom severity [2]. At the 1-year follow-up period, 26 of 32 (81%) surgery patients and 38 of the 68 (56%) in the conservative arm reported good results. Herno et al. reported on 54 matched-pair surgery and conservative treatment patients after a follow-up period that exceeded 4 years [18]. Sixty three percent of 57 surgery patients and 44% of 18 conservative patients, had at least a slight improvement 12 years post-operatively [21]. Using the Oswestry questionnaire and a clinical exam as the outcome measures, the authors did not find a significant difference in subjective disability or functional status between the two groups.
In the current study, 59% of the X STOP and 12% of the NON OP patients were significantly improved in the Symptom Severity and Physical Function domains of the ZCQ questionnaire, and were at least somewhat satisfied with their treatment after 1 year. It is clear that, at 1 year, the X STOP provides pain relief, improved function, and satisfaction in a significantly higher percentage of patients than those treated with conservative measures. Compared with results reported elsewhere, the relatively low level of improvement in the NON OP patients in the current study supports both the rigor of the ZCQ as an outcomes measurement for LSS patients and the efficacy of the X STOP as a treatment modality.
Comparison with reported results of laminectomy
Physical functional and symptom severity improvements for patients treated with the X STOP are comparable with those reported in the literature for patients undergoing a laminectomy or laminotomy, although decompressive surgery was not used as the control in this study. In three reports that used the ZCQ, patients with mild to no back, buttock, and leg pain ranged from 37 to 83%, compared with 64% for X STOP patients at 1 year in the current study (Table 6).
Table 6.
Symptom severity
| Pre-treatment | Post-treatment | |
|---|---|---|
| Severe to very severe pain | No or mild pain | |
| Hansraj et al. [16] | 75 of 77 (97) | 64 of 77 (83) |
| Katz et al. [31] | 213 of 263 (81) | 74 of 199 (37) |
| Simotas et al. (non-op) [48] | 32 of 49 (65) | 12 of 40 (30) |
| Simotas et al. (surgical) [48] | – | 6 of 9 (67) |
| X STOP | 60 of 99 (61) | 57 of 89 (64) |
| NON OP | 53 of 88 (60) | 16 of 78 (21) |
Numbers in parentheses are percentages
These same studies report that the post-operative percentage of patients able to walk 10 blocks ranged from 42 to 73%, compared with 75% of X STOP patients who could walk greater than 2 blocks (Table 7). Twenty-seven percent of X STOP patients could walk greater than 2 miles post-operatively. Despite a slight variation in the way walking distance is measured in the current study, it is clear that the performance of the X STOP patients compares favorably to that of patients undergoing surgical decompression.
Table 7.
Walking distance
| Pre-treatment | Post-treatment | |
|---|---|---|
| Patients unable to walk 2 blocks | Patients able to walk 10 blocks | |
| Hansraj et al. [16] | 48 of 77 (62) | 56 of 77 (73) |
| Katz et al. [31] | 160 of 263 (61) | 84 of 199 (42) |
| Simotas et al. (non-op) [48] | 17 of 49 (35) | 18 of 40 (45) |
| Simotas et al. (surgical) [48] | – | 4 of 9 (44) |
| X STOP | 57 of 99 (58) | 66 of 88 (75) >2 blocks |
| NON OP | 55 of 88 (63) | 39 of 78 (50) >2 blocks |
Numbers in parentheses are percentages
Procedural aspects of X STOP implantation also compare favorably to those reported in the literature for decompressive surgery. The mean operative time for the X STOP procedure was 54 min which was considerably less than the range of 104–224 min reported for laminectomy procedures [4, 22, 39]. In addition, the mean blood loss of 46 cc during the X STOP procedure was less than the range of 120–1040 cc reported for decompressive surgery [4, 22, 39].
Benz et al. reported intra-operative complications such as dural tears and post-operative complications such as wound infection, pneumonia, and epidural hematoma [4]. None of these complications were observed during or after the X STOP procedure. Twenty days after surgery, one X STOP patient in the current study fell and the implant dislodged posteriorly. Another experienced an asymptomatic spinous process fracture that healed without any recurrence of symptoms.
In the current study, the X STOP re-operation rate was 6% (5 of 88), which compares favorably with other reports. Hu et al. reported that the re-operation rate of 4722 patients treated with laminectomy, discectomy, and/or fusion was approximately 10% [20]. Jonsson et al. reported an 18% (19 of 105) re-operation rate of patients treated for LSS [27], and Katz et al. reported re-operation rates of 17% in one report and 23% in another for patients treated for LSS [28, 30].
SF-36
A few studies have reported results from the SF-36 general health questionnaire for patients treated with a lumbar laminectomy. Hozack et al. reported on 41 patients treated for lumbar radiculopathy secondary to disc herniation using the SF-36 questionnaire as an outcomes measure [19]. After a 2-year follow-up, the laminectomy patients showed improvement in all SF-36 sub-scales. Although the patient population in the current study was treated for LSS rather than disc herniation, the SF-36 scores in the X STOP group showed a similar improvement. Finally, Strömqvist et al. used the SF-36 as a general health outcomes measure for patients treated with laminectomy for central and lateral stenosis [52]. Although there were improvements for all sub-scales, they were not as great as those in the X STOP group (Table 5).
Comparisons with other posterior decompressive/stabilization techniques
Several non-fusion spinal implants have been recently introduced which are designed to be implanted from a posterior approach. These implants permit some degree of motion while stabilizing the motion segment in one or more planes. These include the Dynesys [51], DIAM [6], Wallis [45, 46, 47], and FASS [35] systems. Dynesys and FASS are pedicle screw systems with flexible stabilizing rods, whereas the DIAM and Wallis systems are interspinous spacers which incorporate synthetic ligaments that secure the implant to the cranial and caudal spinous processes. Both interspinous implants may be expected to perform in a similar way to the X STOP, except that both will limit flexion to a greater or lesser extent, due to the synthetic ligaments. Results from randomized, prospective studies have thus far not been reported for any of these implants, and the published clinical indications are numerous, including lumbar instability, stenosis, degenerative disc disease, and herniated nucleus pulposus. Data thus far suggest they may be effective in treating some of these lumbar disorders; however, controlled studies are needed to confirm this. The current study, in contrast, clearly demonstrates that the X STOP is safe and effective in treating lumbar spinal stenosis in patients with 1-year follow-up; longer-term follow-up data, although incomplete, confirm the treatment benefit is maintained for at least 2 years.
Limitations
Because non-operative therapy served as a control in the current study, definitive comparisons between the X STOP and decompressive laminectomy cannot be made. Despite this limitation, the current study is one of the more comprehensive evaluations of LSS conducted to date. Turner et al. conducted a meta-analysis of 74 stenosis studies [58], in which the authors noted that no randomized trials comparing surgery to conservative treatment had been conducted. Few studies were prospective, the follow-up data collection methods were unclear, rarely was the data analyzed by someone other than the physician, and the outcomes were not assessed at consistent time intervals. Subsequent clinical studies have somewhat rectified these shortcomings. Care was taken in the current study to address these deficiencies in protocol design. Medical therapy was selected as a control, both because it is so rarely studied in a controlled fashion, and because implantation of the X STOP, like non-operative care, does not require the patient to undergo a highly invasive procedure.
Other limitations are also acknowledged: patient co-morbidities and patient mental state could have been assessed using the Cumulative Illness Rating Scale and the Self Rating Depression Scale respectively to help further explain outcomes. Also, as mentioned in the Results, 64% of the X STOP patients and 48% of the NON OP patients received epidurals before entering the study. Although an epidural steroid injection was a required treatment for patients in the NON OP group, the NON OP patients also received a regimen of other non-operative, patient-specific treatment modalities. The majority of patients were being treated by a general physician before being referred to one of the orthopedic or neurosurgical investigators. Although the treatments the NON OP patients received were not uniform except for at least one required epidural steroid injection, the investigators in this study believe that patients in the NON OP group received a more rigorous and specific regimen of non-operative treatment after they entered the study. Another limitation is a follow-up period of the current study of 1 year. A report will be published based on the 2-year data for all patients.
Despite these limitations, the current study provides evidence that immediate pain relief and increase in function can be provided by the X STOP with a low rate of morbidity. This clinical improvement is significantly greater than that associated with non-operative care, and is similar to success rates attributed to decompressive surgery in the literature.
Conclusion
The results of this prospective controlled study indicate that the X STOP offers a safe and effective treatment for patients with LSS. Implantation of the X STOP can be performed under local anesthesia, and patients without significant comorbidities can return home on the day of surgery. At 1 year, the X STOP provides significant clinical improvement over traditional non-operative therapies, with a success rate comparable to that of published reports for decompressive laminectomy.
Acknowledgement
The authors thank the staff at each of the participating medical centers and St. Francis Medical Technologies for their support.
References
- 1.Amundsen Spine. 1995;20:1178. doi: 10.1097/00007632-199505150-00013. [DOI] [PubMed] [Google Scholar]
- 2.AmundsenSpine 200025142410828926 [Google Scholar]
- 3.Arbit Clin Orthop. 2001;384:137. [PubMed] [Google Scholar]
- 4.Benz Clin Orthop. 2001;384:116. doi: 10.1097/00003086-200103000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Blau Brain. 1978;101:211. doi: 10.1093/brain/101.2.211. [DOI] [PubMed] [Google Scholar]
- 6.Caserta Eur Spine J. 2002;11:S192. doi: 10.1007/s00586-002-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung Skeletal Radiol. 2000;29:217. doi: 10.1007/s002560050596. [DOI] [PubMed] [Google Scholar]
- 8.Ciric J Neurosurg. 1980;53:433. doi: 10.3171/jns.1980.53.4.0433. [DOI] [PubMed] [Google Scholar]
- 9.Deo S, Wanders L, Makan P et al. (1998) Outcome measures for neurogenic claudication. North American Spine Society, San Francisco
- 10.Dong Spine. 1989;14:965. doi: 10.1097/00007632-198909000-00009. [DOI] [PubMed] [Google Scholar]
- 11.DyckSpine 1979489432719 [Google Scholar]
- 12.Dyck J Neurosurg. 1977;46:667. doi: 10.3171/jns.1977.46.5.0667. [DOI] [PubMed] [Google Scholar]
- 13.Fritz Arch Phys Med Rehabil. 1998;79:700. doi: 10.1016/s0003-9993(98)90048-x. [DOI] [PubMed] [Google Scholar]
- 14.Fujiwara Spine. 2001;26:876. doi: 10.1097/00007632-200104150-00010. [DOI] [PubMed] [Google Scholar]
- 15.Gunzburg R, Szpalski M (2000) Lumbar spinal stenosis. Lippincott Williams and Wilkins, Philadelphia, p 414
- 16.HansrajClin Orthop 20013841011249153 [Google Scholar]
- 17.Hasegawa J Bone Joint Surg Am. 1995;77:32. [PubMed] [Google Scholar]
- 18.Herno Br J Neurosurg. 1996;10:461. doi: 10.1080/02688699647087. [DOI] [PubMed] [Google Scholar]
- 19.HozackClin Orthop 1997344889372761 [Google Scholar]
- 20.Hu Spine. 1997;22:2265. doi: 10.1097/00007632-199710010-00013. [DOI] [PubMed] [Google Scholar]
- 21.Hurri J Spinal Disord. 1998;11:110. [PubMed] [Google Scholar]
- 22.Iguchi Spine. 2000;25:1754. doi: 10.1097/00007632-200007150-00003. [DOI] [PubMed] [Google Scholar]
- 23.Inufusa Spine. 1996;21:2412. doi: 10.1097/00007632-199611010-00002. [DOI] [PubMed] [Google Scholar]
- 24.Jenis Spine. 2000;25:389. doi: 10.1097/00007632-200002010-00022. [DOI] [PubMed] [Google Scholar]
- 25.JohnssonClin Orthop 1992279821534726 [Google Scholar]
- 26.Jonsson Spine. 1997;22:2932. doi: 10.1097/00007632-199712150-00016. [DOI] [PubMed] [Google Scholar]
- 27.Jonsson Spine. 1997;22:2938. doi: 10.1097/00007632-199712150-00017. [DOI] [PubMed] [Google Scholar]
- 28.Katz J Bone Joint Surg Am. 1991;73:809. [PubMed] [Google Scholar]
- 29.Katz Arthritis Rheum. 1995;38:1236. doi: 10.1002/art.1780380910. [DOI] [PubMed] [Google Scholar]
- 30.Katz Spine. 1996;21:92. doi: 10.1097/00007632-199601010-00022. [DOI] [PubMed] [Google Scholar]
- 31.Katz Spine. 1999;24:2229. doi: 10.1097/00007632-199911010-00010. [DOI] [PubMed] [Google Scholar]
- 32.Kirkaldy-Willis WH, Wedge JH, Yong-Hing K et al. (1978) Pathology and pathogenesis of lumbar spondylosis and stenosis. Spine:319–328 [DOI] [PubMed]
- 33.Lindsey DP, Swanson KE, Fuchs P et a. (2003) The effects of an interspinous implant on the kinematics of the instrumented and adjacent levels in the lumbar spine. Spine:2192–2197 [DOI] [PubMed]
- 34.Magnaes J Neurosurg. 1982;57:57. doi: 10.3171/jns.1982.57.1.0057. [DOI] [PubMed] [Google Scholar]
- 35.Mulholland Eur Spine J. 2002;11:S198. doi: 10.1007/s00586-002-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onel Spine. 1993;18:291. [PubMed] [Google Scholar]
- 37.Penning Spine. 1987;12:488. doi: 10.1097/00007632-198706000-00013. [DOI] [PubMed] [Google Scholar]
- 38.Porter Spine. 1996;21:2046. doi: 10.1097/00007632-199609010-00024. [DOI] [PubMed] [Google Scholar]
- 39.Postacchini J Bone Joint Surg Br. 1993;75:386. doi: 10.1302/0301-620X.75B3.8496205. [DOI] [PubMed] [Google Scholar]
- 40.Pratt Spine. 2002;27:84. doi: 10.1097/00007632-200201010-00020. [DOI] [PubMed] [Google Scholar]
- 41.Richards J, Majumdar S, Lindsey D et al. (2002) Quantitative changes in the lumbar spinal canal with an interspinous implant. Trans Int Meeting on Advanced Spine Techniques, Montreux
- 42.Saifuddin Clin Radiol. 2000;55:581. doi: 10.1053/crad.2000.0223. [DOI] [PubMed] [Google Scholar]
- 43.Schonstrom J Orthop Res. 1989;7:115. doi: 10.1002/jor.1100070116. [DOI] [PubMed] [Google Scholar]
- 44.Schonstrom Radiol Clin North Am. 2001;39:31. doi: 10.1016/s0033-8389(05)70262-1. [DOI] [PubMed] [Google Scholar]
- 45.Senegas J (1990) La ligametoplastie intervertebrale, alternative a l’arthrodese dans le traitment des instabilities degeneratives. In: Simon L et al. (eds) La hernie discale lombaire. Masson, Paris, pp 308–315
- 46.Senegas Acta Orthop Belg. 1991;57:221. [PubMed] [Google Scholar]
- 47.Senegas Eur Spine J. 2002;11:S164. doi: 10.1007/s00586-002-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simotas Clin Orthop. 2001;384:153. doi: 10.1097/00003086-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 49.Simotas Spine. 2000;25:197. doi: 10.1097/00007632-200001150-00009. [DOI] [PubMed] [Google Scholar]
- 50.Sortland Acta Radiol Suppl. 1977;355:42. [PubMed] [Google Scholar]
- 51.Stoll Eur Spine J. 2002;11:S170. doi: 10.1007/s00586-002-0438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strömqvist Acta Orthop Scand. 2001;72:99. doi: 10.1080/000164701317323327. [DOI] [PubMed] [Google Scholar]
- 53.Stucki J Clin Epidemiol. 1995;48:1369. doi: 10.1016/0895-4356(95)00054-2. [DOI] [PubMed] [Google Scholar]
- 54.Stucki Spine. 1996;21:796. doi: 10.1097/00007632-199604010-00004. [DOI] [PubMed] [Google Scholar]
- 55.Swanson K, Lindsey D, Hsu K et al. (2002) The effect of an interspinous spacer on the intradiscal stress of the adjacent and treated levels. In: Trans Int Meeting on Advanced Spine Techniques, Montreux
- 56.Swanson Spine. 2003;28:26. doi: 10.1097/00007632-200301010-00008. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi K, Hirasawa K, Unayama K (1999) Effects of walking posture, velocity and stride on neurogenic claudication: analysis with epidural pressure. In: Int Soc for the Study of the Lumabr Spine, Hawaii
- 58.Turner Spine. 1992;17:1. doi: 10.1097/00007632-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 59.Verbiest J Bone Joint Surg. 1954;36B:230. doi: 10.1302/0301-620X.36B2.230. [DOI] [PubMed] [Google Scholar]
- 60.Verbiest Clin Neurosurg. 1973;20:204. doi: 10.1093/neurosurgery/20.cn_suppl_1.204. [DOI] [PubMed] [Google Scholar]
- 61.Verbiest Spine. 1976;1:217. [Google Scholar]
- 62.Willen Spine. 1997;22:2968. doi: 10.1097/00007632-199712150-00021. [DOI] [PubMed] [Google Scholar]
- 63.Yerby S, Lindsey D, Swanson K et al. (2002) Influence of an interspinous spacer on the kinematics of the lumbar spine. In: Trans Eurospine. Nantes, France
- 64.Yerby S, Lindsey D, Swanson K et al. (2002) Influence of an interspinous spacer on the kinematics of the lumbar spine. In Trans Int Meeting on Advanced Spine Techniques, Montreux
- 65.Yoshida Spine. 1992;17:1353. doi: 10.1097/00007632-199211000-00015. [DOI] [PubMed] [Google Scholar]
- 66.Zucherman J, Hsu K, Hartjen C et al. (2002) Multicenter randomized prospective trial for treatment of lumbar neurogenic claudication with an interspinous device: 1-year results. In: Trans Int Soc for the Study of the Lumbar Spine, Cleveland, Ohio
- 67.Zucherman J, Hsu K, Hartjen C et al. (2002) Treatment of lumbar spinal stenosis with an interspinous spacer. In: Trans Int Meeting on Advanced Spine Techniques, Montreux
- 68.Zucherman J, Hsu K, Hartjen C et al. (2002) Treatment of lumbar spinal stenosis with an interspinous spacer. In: Trans North American Spine Society, Montreal
- 69.Zucherman J, Hsu K, Hartjen C et al. (2002) Treatment of lumbar spinal stenosis with an interspinous spacer. In: Trans Eurospine. Nantes, France