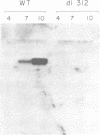
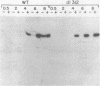
Abstract
Adenovirus mutants carrying a defective E1A gene, such as dl312, are unable to express any of the early viral genes upon infection of HeLa cells. However, efficient expression of the other early adenovirus genes was obtained when dl312-infected HeLa cells were coinfected with pseudorabies virus, a herpesvirus. By employing a temperature-sensitive pseudorabies mutant (tsG1) it was demonstrated that the herpesvirus function responsible for the induction of adenovirus transcription was the immediate early gene, a gene required for the activation of herpesvirus early gene expression and the maintenance of early and late herpesvirus transcription. Specifically, HeLa cells coinfected with dl312 and tsG1, when shifted to the nonpermissive temperature, lost their capacity to express the early adenovirus genes. Furthermore, activation of early adenovirus gene expression in herpesvirus coinfection occurred earlier and at a higher level than in wild-type adenovirus infection. Therefore, the herpesvirus immediate early protein not only activates the early adenovirus transcription units but apparently does so more efficiently than the adenovirus E1A gene product. Because of this fact, we argue that the activation, either by the E1A protein or the herpesvirus immediately early protein, most likely occurs indirectly through interaction with a cellular protein rather than by a direct recognition of regulatory sequences at the adenovirus promoters.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Lee F., Harrison T., Williams J., Sharp P. A. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell. 1979 Aug;17(4):935–944. doi: 10.1016/0092-8674(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Schaffer P. A. Fine-structure mapping and functional analysis of temperature-sensitive mutants in the gene encoding the herpes simplex virus type 1 immediate early protein VP175. J Virol. 1980 Oct;36(1):189–203. doi: 10.1128/jvi.36.1.189-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman L., Rixon F. J., Jean J. H., Ben-Porat T., Kaplan A. S. Transcription of the genome of pseudorabies virus (A herpesvirus) is strictly controlled. Virology. 1979 Sep;97(2):316–327. doi: 10.1016/0042-6822(79)90343-x. [DOI] [PubMed] [Google Scholar]
- Grodzicker T., Anderson C., Sambrook J., Mathews M. B. The physical locations of structural genes in adenovirus DNA. Virology. 1977 Jul 1;80(1):111–126. doi: 10.1016/0042-6822(77)90384-1. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karess R. E., Hanafusa H. Viral and cellular src genes contribute to the structure of recovered avian sarcoma virus transforming protein. Cell. 1981 Apr;24(1):155–164. doi: 10.1016/0092-8674(81)90511-0. [DOI] [PubMed] [Google Scholar]
- Katze M. G., Persson H., Philipson L. Control of adenovirus early gene expression: posttranscriptional control mediated by both viral and cellular gene products. Mol Cell Biol. 1981 Sep;1(9):807–813. doi: 10.1128/mcb.1.9.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Dubbs D. R. Regulation of herpesvirus thymidine kinase activity in LM(TK) cells transformed by ultraviolet light-irradiated herpes simplex virus. Virology. 1977 Jan;76(1):331–340. doi: 10.1016/0042-6822(77)90306-3. [DOI] [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., Schaffer P. A. Thymidine kinase activity of biochemically transformed mouse cells after superinfection by thymidine kinase-negative, temperature-sensitive, herpes simplex virus mutants. Virology. 1978 Apr;85(2):456–463. doi: 10.1016/0042-6822(78)90452-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeMeur M., Glanville N., Mandel J. L., Gerlinger P., Palmiter R., Chambon P. The ovalbumin gene family: hormonal control of X and Y gene transcription and mRNA accumulation. Cell. 1981 Feb;23(2):561–571. doi: 10.1016/0092-8674(81)90152-5. [DOI] [PubMed] [Google Scholar]
- Leiden J. M., Buttyan R., Spear P. G. Herpes simplex virus gene expression in transformed cells. I. Regulation of the viral thymidine kinase gene in transformed L cells by products of superinfecting virus. J Virol. 1976 Nov;20(2):413–424. doi: 10.1128/jvi.20.2.413-424.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W. C., Dimock K., Smiley J. R., Bacchetti S. Herpes simplex virus thymidine kinase transcripts are absent from both nucleus and cytoplasm during infection in the presence of cycloheximide. J Virol. 1980 Nov;36(2):361–365. doi: 10.1128/jvi.36.2.361-365.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Baum P. R., Solem R., Gesteland R. F., Anderson C. W. Location and identification of the genes for adenovirus type 2 early polypeptides. Cell. 1976 Jan;7(1):141–151. doi: 10.1016/0092-8674(76)90264-6. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Nevins J. R. Definition and mapping of adenovirus 2 nuclear transcription. Methods Enzymol. 1980;65(1):768–785. doi: 10.1016/s0076-6879(80)65072-1. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Ginsberg H. S., Blanchard J. M., Wilson M. C., Darnell J. E., Jr Regulation of the primary expression of the early adenovirus transcription units. J Virol. 1979 Dec;32(3):727–733. doi: 10.1128/jvi.32.3.727-733.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Induction of the synthesis of a 70,000 dalton mammalian heat shock protein by the adenovirus E1A gene product. Cell. 1982 Jul;29(3):913–919. doi: 10.1016/0092-8674(82)90453-6. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981 Oct;26(2 Pt 2):213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- Persson H., Monstein H. J., Akusjärvi G., Philipson L. Adenovirus early gene products may control viral mRNA accumulation and translation in vivo. Cell. 1981 Feb;23(2):485–496. doi: 10.1016/0092-8674(81)90144-6. [DOI] [PubMed] [Google Scholar]
- Post L. E., Mackem S., Roizman B. Regulation of alpha genes of herpes simplex virus: expression of chimeric genes produced by fusion of thymidine kinase with alpha gene promoters. Cell. 1981 May;24(2):555–565. doi: 10.1016/0092-8674(81)90346-9. [DOI] [PubMed] [Google Scholar]
- Preston C. M. Control of herpes simplex virus type 1 mRNA synthesis in cells infected with wild-type virus or the temperature-sensitive mutant tsK. J Virol. 1979 Jan;29(1):275–284. doi: 10.1128/jvi.29.1.275-284.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakusanova T., Ben-Porat T., Himeno M., Kaplan A. S. Early functions of the genome of herpesvirus. I. Characterization of the RNA synthesized in cycloheximide-treated, infected cells. Virology. 1971 Dec;46(3):877–889. doi: 10.1016/0042-6822(71)90088-2. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Bishop J. M., Varmus H. E. Glucocorticoid-stimulated accumulation of mouse mammary tumor virus RNA: increased rate of synthesis of viral RNA. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2879–2883. doi: 10.1073/pnas.74.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T., Jones N., Colby W., Fowlkes D. Functional analysis of adenovirus-5 host-range deletion mutants defective for transformation of rat embryo cells. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):367–375. doi: 10.1101/sqb.1980.044.01.041. [DOI] [PubMed] [Google Scholar]
- Swaneck G. E., Nordstrom J. L., Kreuzaler F., Tsai M. J., O'Malley B. W. Effect of estrogen on gene expression in chicken oviduct: evidence for transcriptional control of ovalbumin gene. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1049–1053. doi: 10.1073/pnas.76.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. Y., Roop D. R., Tsai M. J., Stein J. P., Means A. R., O'Malley B. W. Effect of estrogen on gene expression in the chick oviduct. Regulation of the ovomucoid gene. Biochemistry. 1978 Dec 26;17(26):5773–5780. doi: 10.1021/bi00619a026. [DOI] [PubMed] [Google Scholar]
- Van Der Vliet P. C., Levine A. J., Ensinger M. J., Ginsberg H. S. Thermolabile DNA binding proteins from cells infected with a temperature-sensitive mutant of adenovrius defective in viral DNA synthesis. J Virol. 1975 Feb;15(2):348–354. doi: 10.1128/jvi.15.2.348-354.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Clements J. B. A herpes simplex virus type 1 function continuously required for early and late virus RNA synthesis. Nature. 1980 May 29;285(5763):329–330. doi: 10.1038/285329a0. [DOI] [PubMed] [Google Scholar]
- Wiskocil R., Bensky P., Dower W., Goldberger R. F., Gordon J. I., Deeley R. G. Coordinate regulation of two estrogen-dependent genes in avian liver. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4474–4478. doi: 10.1073/pnas.77.8.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]