Abstract
Posterior instrumentation of the cervical spine has become increasingly popular in recent years. Dissatisfaction with lateral mass fixation, especially at the cervico-thoracic junction, has led spine surgeons to use pedicle screws. The improved biomechanical stability of pedicle screws and transarticular C1/2 screws allows for shorter instrumentations and improves the repositioning possibilities. Nevertheless, there are potential risks of iatrogenic damage to the spinal cord, nerve roots or the vertebral artery with both techniques. Therefore, the aim of this study was to evaluate whether C1/2 transarticular screws and transpedicular screws can be applied safely and with high accuracy in the cervical spine and the cervico-thoracic junction using a computer-assisted surgery system (CAS system). Posterior instrumentation was performed using the Brainlab VectorVision System (BrainLAB , Heimstetten, Germany) in 19 patients. Surface matching was used for registration. We placed 22 transarticular screws C1/2, 31 cervical pedicle screws, 10 high thoracic pedicle screws and one lateral mass screw C1. The screw position was evaluated postoperatively using CT with multiplanar reconstruction in the screw axis of each screw. None of the transarticular screws or pedicle screws was significantly (>2 mm) misplaced and no screw-related injury to vascular, neurogenic or bony structures was observed. No screw revision was necessary. The mean operation time was 144 min (90–240 min) and the mean blood loss was 234 ml (50–800 ml). C1/2 transarticular screws, as well as transpedicular screws in the cervical spine and the cervico-thoracic junction, can be applied safely and with high accuracy using a CAS system. Computer-assisted instrumentation is recommended especially for pedicle screws at C3–C6.
Keywords: Computer-assisted surgery, Cervical spine, Posterior instrumentation, Pedicle screws, Transarticular screws, In vivo
Introduction
Transarticular C1/2 screws are widely used in cervical spine instrumentation [15]. The biomechanical stability of C1/2 instrumentation with transarticular C1/2 screw fixation is superior to wiring techniques [13, 14, 38, 47], resulting in lower non-union rates [9]. Nevertheless, there are potential risks of iatrogenic damage to the vertebral artery or the spinal cord [1, 26, 36, 42]. Furthermore, the C1 lateral mass should be fixed properly. This can be difficult to control with the image intensifier [48]. Others have already suggested that the use of a computer-assisted surgery system (CAS system) for transarticular C1/2 screws may be beneficial [29, 41, 44, 46].
The use of pedicle-screw-based spinal instrumentation systems in the lumbar and thoracic spine has increased tremendously in the last decade, due to their superior biomechanical properties and repositioning possibilities [29]. In the lumbar and thoracic spine, conventional screw insertion techniques have been associated with higher screw misplacement rates in cadaver studies, as well as when clinically compared with instrumentations with the aid of a CAS system [6, 10, 16, 23, 24, 30, 31, 33, 35, 39].
Pedicle screws in the cervical spine and the cervico-thoracic junction have become increasingly popular and promising clinical results have been published [2, 4, 29]. Due to improved biomechanical stability of pedicle screws compared with lateral mass screws, pedicle screws allow for shorter instrumentations with improved repositioning capacities [37]. Nevertheless, due to the small size of the cervical pedicles there are potential risks of iatrogenic damage to neural or vascular structures [3, 7, 8, 19, 32].
As in vitro studies have shown that cervical pedicle screws and transarticular screws C1/2 can be safely applied using a CAS system [27, 28, 37], we started using a CAS system intra-operatively for posterior instrumentation of the cervical and cervico-thoracic spine.
The purpose of this study, therefore, was to evaluate the feasibility and accuracy of installing transarticular C1/2 screws, as well as pedicle screws, in the cervical spine and the cervico-thoracic junction, using a CAS system.
Materials and methods
From July to October 2002 the senior author of the paper (M.R.) performed posterior instrumentation of the occipito-cervical, cervical and cervico-thoracic spine in 19 patients using the BrainLAB VectorVision System with Spine 5.0 Software (BrainLAB, Heimstetten, Germany). The Neon Occipito-Cervical System (Ulrich Medizintechnik, Ulm, Germany) was used during the posterior instrumentations.
Preoperative imaging of the patients was done using a helicoidal CT scanner (Somatom Plus 4, Siemens, Germany) with a 1 mm, non-overlapping slice thickness according to the special BrainLAB protocol.
Surface matching was done when using pedicle screws for registration of the instrumented vertebrae; only C2 was matched for transarticular screws C1/2. Twenty-two transarticular screws C1/2 were placed in 12 patients, and 31 cervical pedicle screws, as well as 10 high thoracic pedicle screws, were placed in eight patients. An average of 5.1 (1–8) pedicle screws was used per patient. One C1 lateral mass screw was placed in one rheumatoid patient with C0/C1 instability, a high-riding transverse foramen C2 and spontaneous fusion C1/3. Due to the spontaneous fusion C1/3 surface matching was done on C2 and navigation was possible in C1. The mean widths of the cervical and the high thoracic pedicles were 5.5 mm (4–8 mm) and 6.6 mm (5–8 mm) respectively. The screw position was evaluated postoperatively using CT with multiplanar reconstructions in each screw axis. The screw position of the pedicle screws (PS) was divided into three groups:
Group 1 PS: correct screw placement without pedicle perforation or with pedicle perforation <1 mm
Group 2 PS: pedicle perforation >1 mm without the need for screw revision
Group 3 PS: pedicle perforation >1 mm with the need for screw revision due to irritation or injury of roots or the myelon or due to reduced biomechanical stability.
The screw position of the transarticular screws C1/2 (TAS) was divided into three groups:
Group 1 TAS: correct screw placement without cortical perforation or with cortical perforation <1 mm and appropriate fixation of the lateral mass C1
Group 2 TAS: cortical perforation >1 mm without the need for screw revision
Group 3 TAS: cortical perforation >1 mm or lack of appropriate fixation of the lateral mass C1 with the need for screw revision due to irritation or injury of the myelon or due to reduced biomechanical stability.
The angular deviation between pedicle axis and screw axis, as well as between intra-operative trajectory and screw axis, was measured in postoperative multiplanar CT reconstructions. The evaluation of the postoperative screw positions was done by the third author of the paper, who did not operate on any of the patients by himself but in some cases was a member of the operating team.
Indications for operative therapy, instrumented levels, operative data and patient data are shown in Table 1.
Table 1.
Patients and operative data (TAS transarticular screws, PS4 4.0 mm pedicle screws, PS5 5.0 mm pedicle screws, ML4 4.0 mm lateral mass screws, IS4 4.0 mm isthmic screw C2, l left side, r right side, b both sides)
| Patient no. | Age (years) | Diagnosis | Operation | Placed screws | Operation time (min) | Blood-loss (ml) |
| 1 | 73 | Fracture of the dens | Posterior instrumentation C1/2 with atlas claw | TAS C1/2 b | 100 | 100 |
| 2 | 63 | Rheumatoid instability C1/2 | Posterior instrumentation C1/2 with atlas claw | TAS C1/2 b | 100 | 50 |
| 3 | 66 | Rheumatoid instability C0/2 with spinal stenosis C1 and myelopathy | Posterior instrumentation C0/2 with occiput m-plate, resection of posterior arch C1 | TAS C1/2 b | 120 | 200 |
| 4 | 59 | Progressive kyphosis due to pathological fractures Th1–3 in a case of metastasis of oesophageal cancer with progressive paraparesis | Posterior instrumentation C7/Th5, laminectomy and tumour reduction Th 1–3 | PS4 C7 b; PS5 Th4 b; PS5 Th5 b | 240 | 300 |
| 5 | 65 | Pathological fracture C7 due to metastasis of lung cancer | Posterior instrumentation C6/Th1, laminectomy C7 | PS4 C7 b; PS5 Th1 b | 160 | 250 |
| 6 | 35 | Traumatic multisegmental instability C3/C7 | Posterior instrumentation C3/C7 | PS4 C3 b; PS4 C5 b; PS4 C7 b | 170 | 300 |
| 7 | 49 | Traumatic discoligamentous instability C6/C7 and unstable fracture Th3 | Posterior instrumentation C6/Th4 | PS4 C6 b; PS4 C7 b; PS5 Th2 b; PS5 Th4 b | 180 | 250 |
| 8 | 84 | Non-union of the dens after conservative therapy of an Anderson II dens fracture | Posterior instrumentation C1/2 with atlas claw | TAS C1/2 b | 120 | 100 |
| 9 | 67 | Non-union of the dens after conservative therapy of an Anderson II dens fracture | Posterior instrumentation C1/2 with atlas claw | TAS C1/2 l; PS4 C2 r; ML4 C1 r | 90 | 100 |
| 10 | 71 | Rheumatoid instability C0/2 with spinal stenosis C1 and myelopathy | Posterior instrumentation C0/2 with occiput m-plate, resection of posterior arch C1 | TAS C1/2 b | 180 | 300 |
| 11 | 30 | Post-traumatic instability C0/C2 due to rupture of the alar ligaments | Posterior instrumentation C0/2 with occiput m-plate | TAS C1/2 b | 140 | 100 |
| 12 | 52 | Progressive cervical spondylotic myelopathy C4/C7 | Posterior instrumentation C4/C7, posterior decompression including laminectomy C4/C6 | PS4 C4 b; PS4 C7 b | 200 | 700 |
| 13 | 48 | Progressive rheumatoid instability C1/2 | Posterior instrumentation C1/2 with atlas claw | TAS C1/2 b | 100 | 100 |
| 14 | 63 | Therapy-resistant osteoarthritis of the left C1/2 joint | Posterior instrumentation C1/2 with atlas claw | TAS C1/2 b | 100 | 100 |
| 15 | 84 | Dens fracture Anderson II and unstable Jefferson fracture | Posterior instrumentation C1/2 | TAS C1/2 r; ML4 C1 l; IS4 C2 l | 90 | 50 |
| 16 | 53 | Progressive cervical spondylotic myelopathy C3/C7 | Posterior instrumentation C3/C7, posterior decompression including laminectomy C3/C6 | PS4 C3 b; PS4 C5b; PS4 C7 b | 240 | 800 |
| 17 | 76 | Progressive cervical spondylotic myelopathy C3/C7 | Posterior instrumentation C3/C7, posterior decompression including laminectomy C3/C6 | PS4 C3 b; PS4 C5b; PS4 C7 b | 180 | 400 |
| 18 | Rheumatoid instability C0/C1 (basilar impression) with spontaneous fusion C1/C3 | Posterior instrumentation C0/2 with two single occiput rods | TAS C1/2 r, ML4 C1 l | 110 | 100 | |
| 19 | C1/2 instability and spinal stenosis C1 with progressive myelopathy due to os odontoideum | Posterior instrumentation C0/2 with occiput m-plate, resection of posterior arch C1 | Tas C1/2 b | 130 | 150 |
Neon Occipito-Cervical System
The Neon Occipito-Cervical System is a modular titanium alloy (Ti Al4 V6) implant system (Ulrich Medizintechnik, Ulm, Germany) for posterior instrumentation of the cervical spine and the occipito-cervical and cervico-thoracic junctions. The system consists of 4.5 mm rods, closed connectors of four different lengths and 4.0 mm cannulated, self-tapping and self-drilling screws for C1/2 transarticular instrumentation, 4.0 mm cannulated, self-tapping screws for C2–C7 pedicle instrumentation, as well as lateral mass and transarticular instrumentation from C3 to C7 and 5.0 mm cannulated, self-tapping screws for high thoracic pedicle instrumentation. Cervical fixation is possible with transarticular screws in C1/2, lateral mass screws or transarticular screws from C3 to C7, or pedicle screws from C2 to C7. High thoracic instrumentation is possible with pedicle screws or transverse process screws. The rod-screw connection is constrained.
VectorVision CAS System
The VectorVision System (BrainLAB, Heimstetten, Germany) consists of two calibrated infrared cameras, a high-end workstation with a touch screen monitor, and universal instrument adapters that are attached to trackable probes. The instrument tracking is based on passive marker technology, i.e. infrared-light-emitting diodes are positioned around the cameras. There is a direct line of sight between the cameras and the localisation sensors attached to the surgical instruments, which are wireless and do not require any batteries or electrical power and are thus free to move without power cords. The surgeon can use any instrument after a quick calibration with the Instrument Calibration Matrix (ICM). Navigation is also possible with a pre-calibrated pointing device. Prior to navigation, the patient’s anatomy must be matched intra-operatively to the previously acquired CT data set. The software utilised offers a variety of patient registration methods such as paired point, fluoro to CT and surface matching. During this study surface matching was used.
Surface matching
During the necessary pre-registration of the anteroposterior (AP) direction, one starting point and the head direction must be acquired using the pointer. Following preregistration, a variety of surface points are acquired on the actual patient, which are matched to the CT bone structure surface model using a refined iterative “closest point” algorithm. Generally, up to 20 points are acquired to match the actual spine to the three-dimensional CT image.
Instrument Calibration Matrix (ICM)
The ICM consists of a high-precision manufactured bar with a diameter of 1.5–10 mm as well as a 30 mm diameter opening for specialised instruments. The ICM, which is equipped with reflective marker spheres, is automatically recognised during surgery. The entire registration of any instrument is done in one step and takes less than 1 min. The instrument and ICM are brought into the camera’s view, with the instrument’s tip inserted into the drill hole of the corresponding diameter. The system detects the selected diameter and adjusts the parameters for diameter and vector. Thereafter, the instrument is displayed with the correct diameter and length and is ready for navigation. During the operation, the instrument can be visualised in a three-dimensional surface model and in two-dimensional multiplanar reconstructed images.
Operative techniques
After general endotracheal anaesthesia with muscle relaxation at the start in supine position, the Mayfield clamp is prepped and applied approximately 2 cm above the porus acusticus externus (positioning of the head in a mould without clamp fixation may also be possible, but is not recommended as the possibilities of reduction are limited through the head mould). Then the patient is placed in a prone position on a gel-filled mattress, supporting the thorax and pelvis with foam pillows. Both arms are stabilised through adhesive tape and continuously pulled with 2–3 kg weights. Closed reduction is performed under image intensifier control, if necessary. For C1/C2 transarticular instrumentation, maximal flexion of the occipito-atlantal joints should be achieved while maintaining the reduction. Prepping and draping are performed while maintaining the image intensifier in a lateral plane.
The CAS system is positioned on the right side of the patient once the posterior aspect of the spine has been approached. The monitor with the touchscreen is positioned so that the surgeon has a good view and can easily reach the draped touchscreen. To have a good line of sight, the camera is positioned over the legs of the patient for insertion of pedicle screws and on the right side of the head for transarticular C1/2 screws. The surgeon stands at the head of the patient for inserting pedicle screws and on the left side of the patient for transarticular C1/2 screws.
After a standard midline posterior approach, the reference clamp is fixed to the spinous process of the vertebra intended for instrumentation. Registration of the vertebra is done using the surface matching algorithm, where a predicted accuracy of <1.0 mm was accepted. If the predicted accuracy was >1.0 mm, we repeated the registration procedure until we reached an accuracy of <1.0 mm. After registration of the vertebra, verification was done to ensure that the virtual reality of the CAS system corresponded to the surgical reality. If verification is accurate, the navigated instrumentation can begin; otherwise the entire registration procedure must be repeated.
For pedicle screw instrumentation, we used a navigated drill guide to prepare the pedicle screw holes. In our opinion, the advantage of the drill compared with a pedicle awl is the better feeling of bony resistance due to reduced friction of the rotating drill and the reduced AP forces on the vertebra. In order to avoid bending problems caused by the small stiffness of the 2.6 mm or 3.5 mm drill bit, we tracked the drill guide rather than the drill itself. With this technique, the CAS system can only visualise the trajectory of the drill guide and not the drill itself. Therefore, we used the image intensifier in the lateral view to control the position of the drill in the vertebral body, thereby avoiding perforation of the anterior vertebral body wall. After registration and verification of the proper vertebral level, pedicle screws were prepared with a 2.6 mm drill bit for 4.0 mm screws (Fig. 1) and a 3.5 mm bit for the 5.0 mm screws. After probing the pedicle hole, we inserted the pointer into the pedicle hole to verify the correct position. After the correct position of the pedicle hole was confirmed, we inserted a blunt 1.5 mm k-wire into the vertebral body and the cannulated pedicle screws were implanted over the k-wire. The use of the k-wire reduces the risk of screw malpositioning. Moreover, it also avoids a lateral breakout of the screws from the pedicle, which would endanger the vertebral artery. There is a high risk of lateral breakout of the pedicle screws from C3 to C6, due to the high inclination angle of the cervical pedicle in this region of about 45° and the strong posterior cervical muscles forcing the screwdriver handle to the midline during screw insertion. Due to these factors, we preferred a percutaneous technique for pedicle screw implantation from C3 to C6 using a navigated trocar system, which we inserted over a stab incision. The navigation system was used to find the ideal position for the stab incision, which is difficult to determine without a CAS system (Fig. 2). The orientation of the pedicle holes in C3 and C4 was not chosen in the exact axis of the pedicle, due to the ascending pedicle axis in these vertebrae. The entrance point was chosen at the upper margin of the pedicle with a descending direction, so that the drill would not perforate the upper endplate of the vertebra and the longest possible screw length was selected.
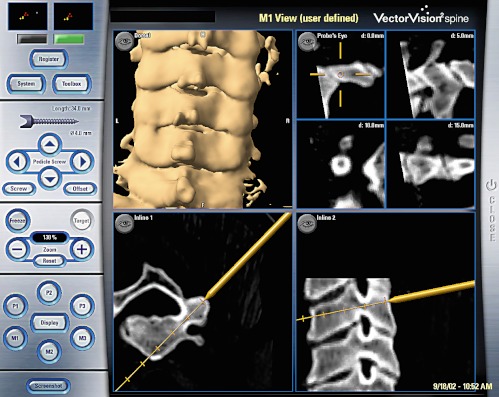
Fig. 1.
Screenshot taken during preparation of the right pedicle C3 using the 2.6 mm drill in a percutaneous technique with the navigated trocar system
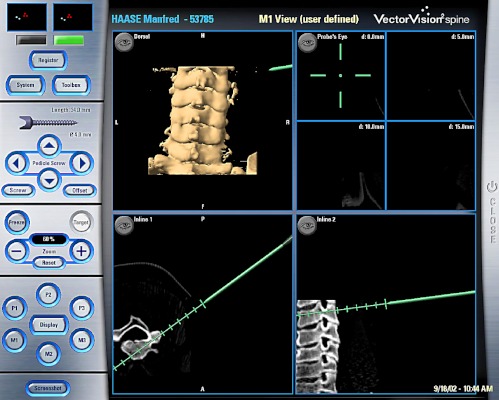
Fig. 2.
Screenshot taken while defining the position of the stab incision for the trocar system for pedicle screw instrumentation C3
The C1/2 transarticular instrumentation was performed with a sharp 1.5 mm k-wire and the above-mentioned trocar system for percutaneous k-wire and screw application, which was tracked by the CAS system. The 1.5 mm k-wire was suitable for use with the cannulated 4.0 mm self-drilling and self-tapping neon screws. This type of instrumentation has a distinct adantage compared with other methods, in that no dislocation between C1 and C2 can occur during insertion of the screw, as may occur after removing a drill when using a non-cannulated system, especially in severe instabilities. Furthermore, the length of the posterior approach can be reduced due to the percutaneous insertion of the drill guide using high thoracic stab incisions. Similarly to the navigation of the drill for pedicle screws, the CAS system can only visualise the trajectory of the k-wire and not the k-wire itself. Therefore, we used the image intensifier in the lateral view to control the position of the k-wire in C2. As the instruments could only be navigated in C2 when using the CAS system, the image intensifier was also necessary to control the repositioning of C1/2 (if necessary) and to control the k-wire position in C1. After inserting the trocar system the 1.5 mm sharp k-wire was installed according to the recommendations of Magerl (Fig. 3). As the k-wire can stray from the proposed trajectory in the frontal plane as well if the density of the cortical bone is very high, we used the image intensifier in the AP view after installing both k-wires to ensure a correct k-wire position. After insertion of the transarticular screw C1/2 over the k-wire and through the trocar system, the k-wire was removed. The trocar should not be removed before the end of the operation because of possible bleeding from the trocar canal.
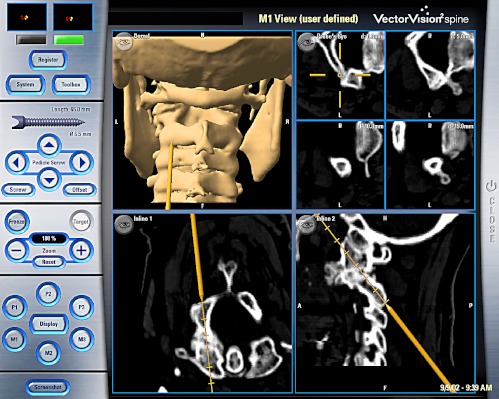
Fig. 3.
Screenshot during the action of installing the sharp 1.5 mm k-wire for the cannulated self-drilling transarticular screws C1/2 in a percutaneous technique with the navigated trocar system
Results
The mean operation time was 144 min (90–240 min) and the mean blood loss was 234 ml (50–800 min). No postoperative neurological deterioration was observed. A deep wound infection occurred in one patient with a rheumatoid instability C0/C2 and methotrexate as well as corticoid medication, which was successfully treated by debridement without implant removal. No other intra- or postoperative complications occurred.
Thirty-one of 31 cervical pedicle screws and 10 of 10 high thoracic pedicle screws were implanted using the CAS system as planned preoperatively. Thirty of 31 cervical (97%) and all high thoracic pedicle screws (100%) were graded in group 1 PS (Fig. 4). The mean screw angle was 38.9° (27°–55°) and the mean pedicle angle was 40.6° (27°–55°) for the cervical pedicle screws, and 18.9° (10°–32°) and 19.0° (10°–36°) respectively for the high thoracic pedicle screws. One cervical pedicle screw (3%) in C5 was graded in group 2 PS due to a lateral pedicle perforation of 1.6 mm without injury to the vertebral artery. This pedicle was mainly cortical bone with a diameter of 4.0 mm. As the screw was placed correctly in the vertebral body, no revision of this screw was necessary. No screw-related injury to vascular, neurogenic or bony structures was observed with the screws graded in group 1 PS. No screw revision of the screws graded in group 1 PS was necessary. The mean angular deviations between intra-operative screw trajectory and postoperative screw position for the pedicle axis and screw axis were 2.4° (0°–9°) and 3.0° (0°–10°) respectively.
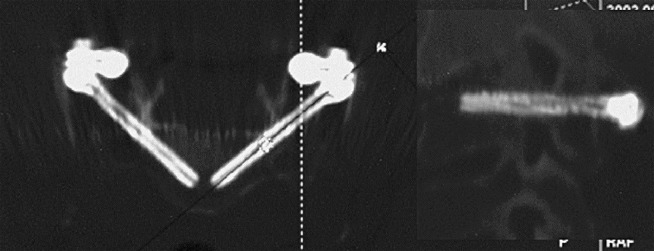
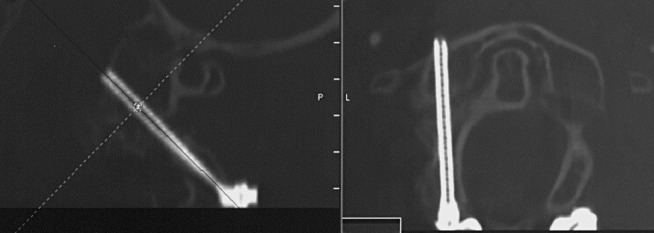
Fig. 4.
Postoperative CT control of C3 pedicle screws with multiplanar reconstructions in the screw axis
Twenty of 22 transarticular screws were implanted using the CAS system as planned preoperatively. In one patient with very dense cortical bone the k-wire did not perforate the cortical wall at the end of the C2 isthmus and, therefore, followed the inner wall of the isthmus, which led to bending of the k-wire on the left side in the frontal plane. This bending of the k-wire was detected on the image intensifier in the lateral view. In this case the k-wire was installed using the image intensifier in two planes without the CAS system. In one other patient, with rheumatoid instability at C1/2, a complete reduction C1/2 in the frontal plane was not possible. Therefore the image intensifier was used in the AP and lateral views to control k-wire placement in the lateral mass of C1 on both sides and the CAS system was used to place the k-wires through the isthmus of C2.
The position of all transarticular screws C1/2 in the postoperative CT scans corresponded to the intra-operative trajectory documented by intra-operative screenshots. All transarticular screws C1/2 were graded in group 1 TAS (Fig. 5). None of the transarticular screws C1/2 were misplaced, and no screw-related injury to vascular, neurogenic or bony structures was observed. None of the screws perforated the cortex near the groove of the vertebral artery in C2. No screw revision was necessary.
Fig. 5.
Postoperative CT control of transarticular screws C1/2 with multiplanar reconstructions in the screw axis
The lateral mass screw C1 in the one rheumatoid patient with a high-riding transverse foramen and spontaneous fusion C1/3 was also placed correctly, without bony perforation of the lateral mass.
Discussion
Although pedicle instrumentation is very common in the lumbar and thoracic spine, it is still uncommon in the cervical spine. The small dimensions of the cervical pedicle, and the proximity of vascular and neural structures, may explain this. The main posterior instrumentation technique in the cervical spine, i.e. lateral mass screw fixation, results in a biomechanical stability clearly below that achieved with pedicle screws [19, 22]. Nevertheless there are potential risks of iatrogenic damage to neural or vascular structures [3, 7, 8, 19, 32].
Due to the advantages of cervical and high thoracic pedicle screws over other instrumentation techniques, the interest of spine surgeons in this technique is increasing rapidly [2, 4, 29]. As a result of improved biomechanical stability of pedicle screws compared with lateral mass screws, pedicle screws allow for shorter instrumentations with improved repositioning capacities. Although excellent results with very low screw misplacement rates were published by Abumi et al. [3], demonstrating a 7% misplacement rate out of 669 cervical pedicle screws when using a conventional screw insertion technique without a CAS system, these data may not be applicable to many other spine surgeons with significantly less experience in cervical pedicle screws.
In 1993, CAS systems were developed for the installation of pedicle screws in the lumbar spine [5, 25, 33] based on reports of misplacement rates between 5% and 40% using conventional techniques [11, 12, 17, 18, 40, 43, 45]. In vitro studies showed that misplacement rates of pedicle screws can be significantly reduced when using CAS systems [6, 21, 34, 35]. In vivo studies confirmed these results [20, 24, 30, 31, 39]. As in vitro studies have shown that cervical pedicle screws and transarticular screws C1/2 can be safely applied using a CAS system [27, 28, 37], we started using a CAS system intra-operatively for posterior instrumentation of the cervical and cervico-thoracic spine.
We decided to use a drill to prepare the pedicles due to the reduced friction of a rotating drill compared with a pedicle awl. As 2.6 mm drills bend significantly during drilling, resulting in reduced accuracy, we designed a drill guide suitable for the CAS system. The results of our in vitro study [37] done with the Navitrack CAS system (Orthosoft, Montreal, Canada) show that this technique is suitable for cervical pedicle instrumentation. In this study we had a perforation rate of 8% without harm to vascular or neural structures. The two minor pedicle perforations, in our in vitro study in pedicles with a width below 4.0 mm, point out possible anatomical restrictions. The single cervical pedicle screw in our present in vivo study graded in group 2 PS also underlines this. The width of this pedicle was 4.0 mm.
Pedicle width of C3 averages 4.9 mm in males and 4.5 mm in females, the minimum reported width being 3.0 mm. C4 averages 4.7 mm in males and 4.6 mm in females, with a minimum reported width of 3.1 mm [8]. The pedicle width in C5 and C6 is slightly higher. These anatomical data show that some pedicles may not be suitable for pedicle screws. Therefore, pedicle width should be measured preoperatively using CT. Although the risk associated with pedicle screws C3–C6, is obviously higher compared with C7 and high thoracic pedicle screws, the use of a CAS system for C7 and high thoracic pedicle screws may also be beneficial, as the quality of the intra-operative image intensifier picture in this region is usually rather poor due to the shoulders.
In contrast to pedicle instrumentation, transarticular screw fixation in C1/2 is already very common in cervical spine surgery [15]. The biomechanical stability of C1/2 instrumentation with transarticular C1/2 screw fixation is superior to wiring techniques [13, 14, 47], resulting in lower non-union rates [9]. When using the CAS system, only C2 can be registered, rendering computer navigation possible only in C2; thus the image intensifier was also necessary to control the repositioning of C1/2 (if necessary) and to control the k-wire position in C1. As the k-wire can stray from the proposed trajectory in the frontal plane as well if the density of the cortical bone is very high, we used the image intensifier in the AP view after installing both k-wires to ensure a correct k-wire position. Due to the facts mentioned above, transarticular instrumentation C1/2 with the CAS system cannot be performed without an image intensifier, at least in the lateral view. In spite of the above-mentioned problems with computer-assisted instrumentation for transarticular screws C1/2, the use of a CAS system for transarticular screws C1/2 seems to be beneficial in ensuring a correct screw position in C2. Furthermore, the exact positioning of the stab incisions for the percutaneous technique is a huge advantage compared with conventional techniques.
Although a CAS system is a useful and fascinating tool for spine surgery, anyone using such a system should be aware of possible errors. As the system can crash at any time during the operation due to hardware, software or human failure, every surgeon using the system should be experienced in the operation without the use of a CAS system. Furthermore, the surgeon should never rely on the virtual information he or she receives from the CAS system without their own verification. The surgeon must ensure that the correct vertebrae are instrumented via image intensifier control or other techniques. Although registration of the instrumented vertebrae was possible in all cases, it can be difficult especially in C3 and C4, as these vertebrae have very small spinous processes and in many patients the posterior surface of the vertebrae, which is used for the surface matching, is quite similar. Therefore, it is possible to achieve an acceptable registration on C3 with the surface data of C4 or vice versa. This problem underlines the mandatory need for the surgeon to verify that he or she instruments the correct vertebra. Another error may result from the reference clamp, which is attached to the vertebra on which the surgeon will be working. In the middle of the cervical spine, the small dimensions of the spinous processes makes it difficult to achieve a stable fixation of the reference clamp, especially as most of the reference clamps available for the different CAS systems were initially designed for the lumbar and thoracic spine. Adapted reference clamps or other fixation techniques for the reference marker star should be developed for the cervical spine anatomy.
Conclusions
C1/2 transarticular screws, as well as transpedicular screws in the cervical spine and the cervico-thoracic junction, can be applied safely and with high accuracy using a CAS system. A CAS system is recommended, especially for pedicle instrumentation C3–C6, due to the potential risk of injury to the vertebral artery. The use of a CAS system for posterior cervical spine surgery will make a good spine surgeon even better, but it can never make a bad spine surgeon a good one. Lars Leskell’s quotation from 1994 “A fool with a tool is still a fool” is therefore a good motto to adopt for the use of CAS systems in spine surgery. It is of the utmost importance that the surgeon is experienced and able to perform the surgery without the CAS system as well.
References
- 1.Abou J Bone Joint Surg Br. 1997;79:820. doi: 10.1302/0301-620x.79b5.7566. [DOI] [PubMed] [Google Scholar]
- 2.Abumi Spine. 1997;22:1853. doi: 10.1097/00007632-199708150-00010. [DOI] [PubMed] [Google Scholar]
- 3.Abumi Spine. 2000;25:962. doi: 10.1097/00007632-200004150-00011. [DOI] [PubMed] [Google Scholar]
- 4.AbumiJ Neurosurg 2000923010616055 [Google Scholar]
- 5.Amiot Spine. 1995;20:1208. doi: 10.1097/00007632-199505150-00019. [DOI] [PubMed] [Google Scholar]
- 6.Berlemann J Spinal Disord. 1997;10:117. [PubMed] [Google Scholar]
- 7.Ebraheim Spine. 1996;21:691. doi: 10.1097/00007632-199603150-00005. [DOI] [PubMed] [Google Scholar]
- 8.Ebraheim Spine. 1997;22:1. doi: 10.1097/00007632-199701010-00001. [DOI] [PubMed] [Google Scholar]
- 9.Farey Clin Orthop. 1999;359:126. doi: 10.1097/00003086-199902000-00013. [DOI] [PubMed] [Google Scholar]
- 10.GeorgeSpine 1991161812011773 [Google Scholar]
- 11.GertzbeinSpine 199015112326693 [Google Scholar]
- 12.Glossop Spine. 1996;21:2026. doi: 10.1097/00007632-199609010-00021. [DOI] [PubMed] [Google Scholar]
- 13.Grob Spine. 1992;17:480. doi: 10.1097/00007632-199205000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Grob D, Dvorak J, Panjabi MM, Hayek J (1991) Die dorsale atlantoaxiale Verschraubung. Ein Stabilitätstest in vitro und in vivo. Orthopäde 20:154–162 [PubMed]
- 15.Grob J Bone Joint Surg Br. 1991;73:972. doi: 10.1302/0301-620X.73B6.1955447. [DOI] [PubMed] [Google Scholar]
- 16.Jeanneret J Spinal Disord. 1990;3:413. [PubMed] [Google Scholar]
- 17.Jerosch Z Orthop. 1992;130:479. doi: 10.1055/s-2008-1039656. [DOI] [PubMed] [Google Scholar]
- 18.JiaChung Hua Wai Ko Tsa Chih 1989275612630231 [Google Scholar]
- 19.Jones Spine. 1997;22:977. doi: 10.1097/00007632-199705010-00009. [DOI] [PubMed] [Google Scholar]
- 20.Kalfas J Neurosurg. 1995;83:641. doi: 10.3171/jns.1995.83.4.0641. [DOI] [PubMed] [Google Scholar]
- 21.Kamimura J Orthop Sci. 1999;4:197. doi: 10.1007/s007760050094. [DOI] [PubMed] [Google Scholar]
- 22.Kotani Spine. 1994;19:2529. doi: 10.1097/00007632-199411001-00007. [DOI] [PubMed] [Google Scholar]
- 23.Laine Eur Spine J. 1997;6:402. doi: 10.1007/BF01834068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laine Spine. 1997;22:1254. doi: 10.1097/00007632-199706010-00018. [DOI] [PubMed] [Google Scholar]
- 25.Lavallee J Image Guid Surg. 1995;1:65. doi: 10.1002/(SICI)1522-712X(1995)1:1<65::AID-IGS10>3.3.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 26.Lu Spine. 1998;23:1229. doi: 10.1097/00007632-199806010-00011. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig Spine. 2000;25:2675. doi: 10.1097/00007632-200010150-00022. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig Spine. 2000;25:1655. doi: 10.1097/00007632-200007010-00009. [DOI] [PubMed] [Google Scholar]
- 29.Ludwig SC, Kramer DL, Vaccaro AR, Albert TJ (1999) Transpedicle screw fixation of the cervical spine. Clin Orthop 77–88 [DOI] [PubMed]
- 30.Merloz Clin Orthop. 1998;354:39. doi: 10.1097/00003086-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Merloz Comput Aided Surg. 1998;3:297. doi: 10.1002/(SICI)1097-0150(1998)3:6<297::AID-IGS3>3.3.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 32.Miller Spine. 1996;21:2317. doi: 10.1097/00007632-199610150-00003. [DOI] [PubMed] [Google Scholar]
- 33.Nolte Stereotact Funct Neurosurg. 1996;66:108. doi: 10.1159/000099677. [DOI] [PubMed] [Google Scholar]
- 34.NolteSpine 1995204977747237 [Google Scholar]
- 35.Nolte Clin Biomech. 1995;10:193. doi: 10.1016/0268-0033(95)00004-5. [DOI] [Google Scholar]
- 36.Paramore J Neurosurg. 1996;85:221. doi: 10.3171/jns.1996.85.2.0221. [DOI] [PubMed] [Google Scholar]
- 37.Richter Eur Spine J. 2000;9:S65. doi: 10.1007/PL00010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richter Spine. 2002;27:1724. doi: 10.1097/00007632-200208150-00008. [DOI] [PubMed] [Google Scholar]
- 39.Schwarzenbach Spine. 1997;22:452. doi: 10.1097/00007632-199702150-00020. [DOI] [PubMed] [Google Scholar]
- 40.Sim Acta Orthop Scand. 1993;64:28. doi: 10.3109/17453679308994522. [DOI] [PubMed] [Google Scholar]
- 41.Solanski Spine. 1999;14:1477. doi: 10.1097/00007632-199907150-00014. [DOI] [PubMed] [Google Scholar]
- 42.Vaccaro Spine. 1994;19:2637. [PubMed] [Google Scholar]
- 43.Vaccaro J Bone Joint Surg Am. 1995;77:1200. doi: 10.2106/00004623-199508000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Weidner Spine. 2000;25:2668. doi: 10.1097/00007632-200010150-00020. [DOI] [PubMed] [Google Scholar]
- 45.Weinstein Spine. 1988;13:1012. doi: 10.1097/00007632-198809000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Welch Neurosurgery. 1997;40:958. doi: 10.1097/00006123-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 47.Wilke Eur Spine J. 1992;1:191. doi: 10.1007/BF00301312. [DOI] [PubMed] [Google Scholar]
- 48.Xu Spine. 1998;23:2190. doi: 10.1097/00007632-199810150-00009. [DOI] [PubMed] [Google Scholar]