Abstract
Background
Radiological changes and degeneration of the cervical spine have been previously described in soccer players. The onset of such changes was 10–20 years earlier than that of the normal population. The aim of this study was to assess these early degenerative changes in amateur active and veteran soccer players in a cross-sectional descriptive study using biomechanical, radiological, and magnetic resonance measures.
Methods
The subjects were active (<30 years; n=15) and veteran (>30 years; n=15) male amateur soccer players, and their age-matched controls (n=13 and n=15). Biomechanical measurements were made on a cervical dynamometer. Dynamic radiological and magnetic resonance findings were also obtained and evaluated.
Results
The normalized mean extension moment was higher in the active soccer players, but the mean range of motion was lower. Degenerative changes were prominent in veteran players, and the sagittal diameter of their spinal canal at C2 to C6 was lower when compared to active players and controls. Magnetic resonance findings of degeneration were more prominent in soccer players when compared to their age-matched controls.
Conclusion
A tendency towards early degenerative changes exists in soccer players most probably due to high- and/or low-impact recurrent trauma to the cervical spine caused by heading the ball.
Keywords: Cervical spine, Soccer, Low-impact recurrent trauma, Biomechanics, Radiology, Magnetic resonance
Introduction
Soccer is one of the most popular sports around the globe with a high incidence of trauma [5, 7, 14]. Brain injuries [9, 16, 22] in soccer are well defined, but those of the cervical spine are rarely determined. Cervical spinal injuries are usually categorized as “head and neck injuries”, and their incidence varies from 4 to 22% [10, 24]. Severe muscle spasms, spinal cord injuries [21], disc herniation and fracture dislocations [23] are documented among the acute injuries to the cervical spine in soccer. Apart from direct and indirect trauma to the cervical spine, scoring, defending and passing the ball with the head is an integral part of this game; so chronic degenerative changes should be common in the cervical spine. However, the effect of high- and/or low-impact recurrent trauma to the cervical spine in soccer is rarely recognized [23].
It is hypothesized that high- and/or low-impact recurrent trauma mainly due to heading the ball may initiate degenerative changes at the cervical spine. With the co-existence of a cervical narrow canal [6], these changes may predispose factors for developing cervical spondylosis. Such a co-existence would produce pressure on the spinal cord and may make soccer players more susceptible to neurological complications. Abnormal radiological findings have been described in amateur veteran soccer players [11], however, biomechanical studies or advanced diagnostic evaluation using magnetic resonance (MR) were not carried out to quantify degeneration and existing pathologies.
The aim of this cross-sectional descriptive study was to determine the effects of playing soccer on the cervical spine using biomechanical, radiological and MR measures.
Subjects and methods
Subjects were active (S1 group; n=15) and veteran (S2 group; n=15) male soccer players. The S1 group comprised amateur soccer players of a university team under the age of 30 years and the S2 group comprised veterans over the age of 30 years who had played active soccer for at least 10 years. The K1 (n=13) and K2 (n=15) groups were the age-matched controls of the S1 and S2 groups. The control subjects of the K1 and K2 groups were randomly selected among university staff and students. These subjects did not participate in regular sports, and their weekly physical activity level was less than 45 min/day and 3 days/week. A written consent was given to the subjects before the tests to inform them about the procedures, and their permission was obtained for the biomechanical, radiological and MR evaluations. Body weight and height were measured and the body mass index (BMI) was calculated.
Biomechanical tests
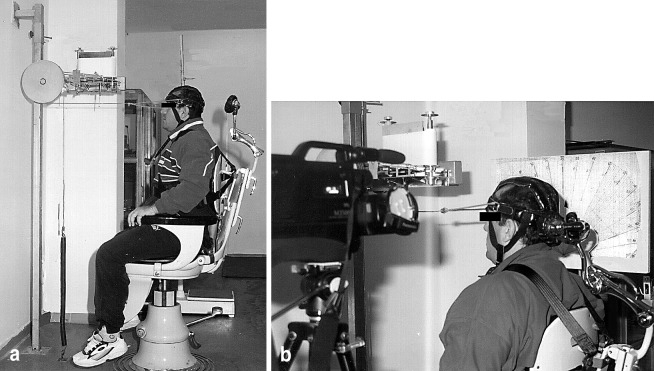
A cervical dynamometer was created according to information derived from the literature (Fig. 1) [1, 18]. This dynamometer allowed cervical flexion and extension only, and limited other parts of the body from interfering with this motion. Cervical extension moments and strengths were measured by this dynamometer. The chair of the dynamometer on which the subject was sitting was adjusted according to the height of the individual. Chest straps were applied to minimize external vertical, lateral and rotational forces of the corpus on the cervical spine. Repetition time of cervical flexion and extension was recorded automatically by a mechanical counter mounted on the dynamometer. A 50-Hz videocamera recorded the range of motion (ROM) from a visual scale adjusted to the side of the dynamometer, and was level with the head and cervical spine.
Fig. 1.
Biomechanical setup for the measurement of normalized work (joules), normalized power (watts) and normalized moment (N/m)
A spring with a constant resistance was fitted to the ground on one side of the dynamometer. The other side of the spring was mounted to a steel rope that was placed anteriorly to the headgear worn by the subject. The steel rope passed over the pulley system of the dynamometer. Prior to the tests, the subject was asked to stretch his neck to the maximal extent. The head stop at the back of the chair was locked at this position and displacement of the spring was measured. The subject was then asked to perform a maximal flexion to extension motion for 1.5 min. Repetition of the last 1 min was counted and recorded for each subject. Work, power and mean extension moments were calculated from these recordings using the formulae given below:
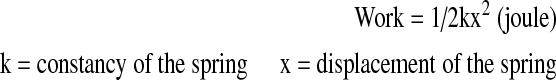 |
1 |
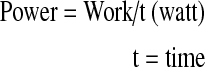 |
2 |
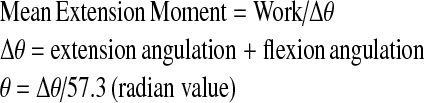 |
3 |
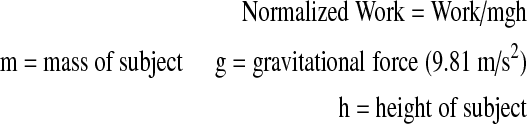 |
4 |
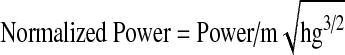 |
5 |
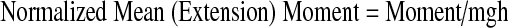 |
6 |
The curve angulation’s K constant of the spring was measured by the least squares method from the lengthening function towards force using Excel 4.0 for Windows software on a personal computer.
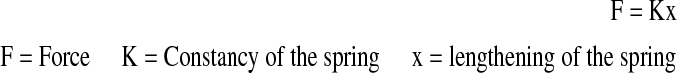 |
7 |
The reliability of biomechanical testing was confirmed by re-testing the same subjects after 15 days. Findings were analyzed using the Pearson correlation technique. The correlation coefficient of repetitive measurement was 0.87.
Calculation of Δt
A motion analyzer at a setting of 1/25 s−1 was used to measure the time spent for flexion and extension. The flexion/extension ratio was 1/1. At the end of 1 min of exercise, 30 s was used to pull the spring while 30 s was used for relaxation.
Radiological measurements
Lateral cervical radiographs at neutral, flexion and extension positions were obtained while the subject was standing in a neutral upright position, avoiding the straightening of the spine. A Siemens Multix C machine was used. The distance of the X-ray source to the subject was 100 cm and the setting was 63–66 kV and 32 mAs. Degenerative changes were evaluated according to Dihlmann [2] and graded from 0 to 5.
Radiological parameters assessed were: (a) dislocation, (b) ankylosis, (c) osteochondrosis, (d) osteolysis, (e) antero-posterior angulation, (f) arthrosis, (g) platyspondylosis, (h) kyphosis, (i) loss of flexibility, (j) translation, (k) anomaly, (l) pedicular enlargement and (m) enlargement of facet joints. Kinematic analyses on radiograms were made using the Beuetti-Bauml and Panjabi techniques [25]. The sagittal diameter of the spinal canal at C2 to C6 was also measured.
Magnetic resonance
A 1.5 T MR (Siemens Magneton Vision Plus, Erlangen, Germany) with a standard protocol was used (Table 1). The average duration of evaluation was 20 min. Bone and intervertebral disc pathologies (bulging, osteophytes, disc protrusion), loss of cervical lordosis, spinal cord impingement (static and dynamic), spinal cord compression in hyperflexion, and volume of transversospinal muscles (semispinalis cervicis and semispinalis capitis) were measured (Fig. 2).
Table 1.
Magnetic resonance protocol (TR time to repetition, TE time to echo, FA flip angle, TSE turbo-spin echo, GRE gradient re-called echo)
| Sequence | Plan | TR/TE (ms)/FA (degrees) | Time (min) |
|---|---|---|---|
| Scout | Various | 25/6/30 | 0.14 |
| TSE T2-weighted | Sagittal | 4000/112/180 | 3.16 |
| TSE T2-weighted | Coronal | 4000/112/180 | 3.16 |
| GRE T2-weighted | Transverse | 750/22/30 | 4.21 |
| TSE T1-weighted | Sagittal | 100/12/180 | 0.53 (kinematic 4 series) |
Fig. 2.
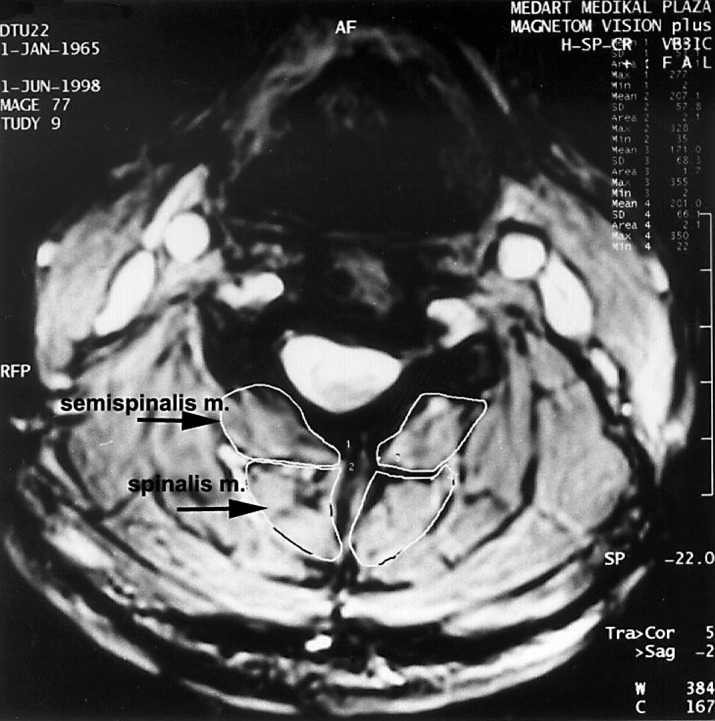
Manual edge determination was used to measure the volume of transversospinal muscles (semispinalis capitis and semispinalis cervicis)
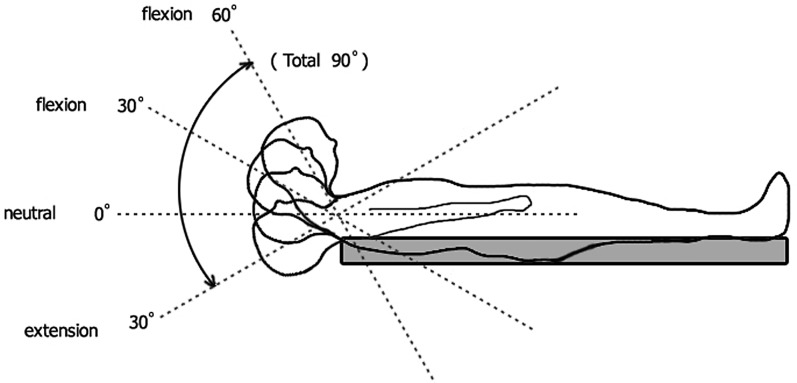
Kinematic analysis was carried out in a classical, closed MR. The cervical spine of each subject was imaged four times by placing pre-prepared triangle-shaped polyethylene cushions under the neck. These cushions stabilized the neck at 30° extension, neutral, 30° and 60° flexion positions while the subject was lying in the MR device in the supine position. Dynamic spinal cord compression from extension to flexion (overall 90°) was evaluated by this method (Fig. 3). Independent radiologists who did not know the groups of subjects evaluated the radiological and MR findings.
Fig. 3.
Kinematic MR analysis for the assessment of dynamic spinal cord compression from extension to flexion in a classical, closed MR
Statistical analysis
SPSS software was used for statistical analyses. One-way variance analysis (ANOVA) was performed to compare the results of the soccer players and controls, whereas the post-hoc test was used to define the variations between the groups.
Results
Age, weight, height and BMI values of the subjects are presented in Table 2. The only significant difference was between the height of the subjects among the S1 and K1 groups. Weight and BMI values were comparatively similar among all groups.
Table 2.
Information on subjects includes age, weight, height, body mass index (BMI), number of years spent in active soccer activity (NY), hours of training a week (HTW) and matches per year (M/Y)
| Group | n | Age | Weight (kg) | Height (cm) | BMI (kg/m2) | NY | HTW | M/Y |
|---|---|---|---|---|---|---|---|---|
| S1 | 15 | 24.6±0.5 | 74.8±1.2 | 179.0±7.7 | 23.6±1.3 | 12.5±1.6 | 6.4±0.9 | 27.8±4.3 |
| K1 | 13 | 20.6±1.7 | 70.6±9.7 | 172.3±6.6 | 23.7±2.4 | - | - | - |
| S2 | 15 | 35.0±3.4 | 76.2±5.2 | 175.8±4.7 | 24.6±1.5 | 18.0±0.8 | 6.1±1.0 | 28.7±4.0 |
| K2 | 15 | 38.3±4.7 | 74.3±8.6 | 173.1±5.2 | 24.8±2.9 | - | - | - |
| F | - | - | 1.46 | 5.87 | 1.73 | - | - | - |
| P | - | - | 0.23 | 0.002 | 0.17 | >0.01 | 0.18 | 0.29 |
Biomechanical findings
The normalized mean extension moment was higher in the S1 group when compared to other groups (p=0.056). Normalized work (p=0.012) and normalized power (p=0.008) were significantly different between the S1 and S2 groups. Normalized power (but not normalized work) was significantly different (p=0.011) between the K1 and K2 groups (Table 3).
Table 3.
Biomechanical test results in means of normalized work (joules), power (watts), and moment (N/m)
| Group | Normalized work (J) | Normalized power (W) | Normalized moment (N/m) |
|---|---|---|---|
| S1 | 0.033±0.01 | 0.029±0.01 | 2.32±0.29 |
| K1 | 0.027±0.01 | 0.022±0.01 | 1.92±0.38 |
| S2 | 0.027±0.01 | 0.021±0.00 | 2.09±0.38 |
| K2 | 0.026±0.01 | 0.017±0.01 | 1.73±0.46 |
| F | 4.04 | 4.33 | 2.62 |
| P | 0.012 | 0.008 | 0.056 |
ROM of the cervical spine
The average ROMs of the S1, S2, K1 and K2 groups were 104.7±1.5, 96.3±1.4, 109.0±2.1 and 107.5±2.3, respectively. The ROM of the S1 group was significantly lower than that of the K1 group (f=8.10; p=0.01).
Radiological findings
The intersegmentary angulation of the C2–C3 level was significantly lower in the K1 group when compared to the K2 group (f=3.59; p=0.02), but not between the S1 and S2 groups. Intersegmentary angulation of other levels was not significantly different when all groups were compared. Degenerative changes were prominent in the S2 group when compared to the S1 and K2 groups, however, they were not statistically significant. The sagittal diameter of the spinal canal was lower in the S2 group when compared to the S1 group, but this was only significant at the C5 level (f=3.02; p=0.02) (Fig. 4; Table 4).
Fig. 4.
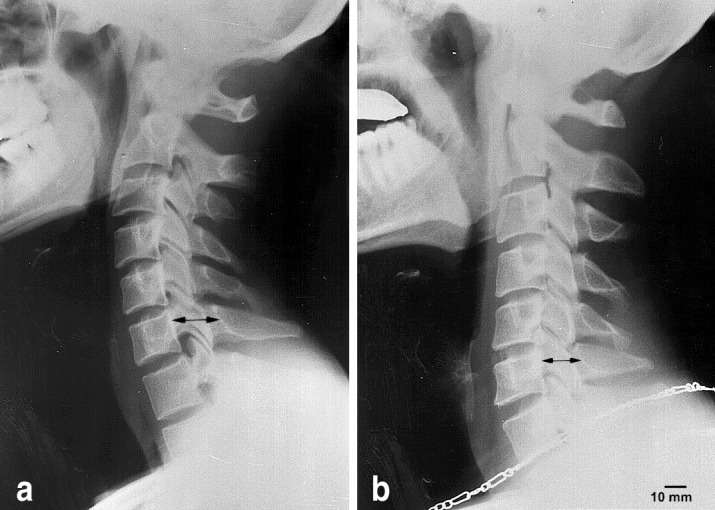
Lateral neutral cervical radiography of a sample subject of the S1 (a) and S2 (b) groups. The spondylotic anteroposterior diameter was significantly lower in the S2 group when compared to the S1 group (p=0.03)
Table 4.
Saggital diameter (mm) of the spinal canal between C2 and C6
| Group | C2 | C3 | C4 | C5 | C6 |
|---|---|---|---|---|---|
| S1 | 18.7±1.7 | 18.1±1.9 | 18.5±2.8 | 19.3±1.8 | 18.2±1.5 |
| K1 | 17.6±2.1 | 17.3±2.6 | 16.9±2.9 | 17.2±2.5 | 17.3±2.6 |
| S2 | 18.2±2.0 | 17.8±2.5 | 17.7±2.0 | 17.9±1.9 | 18.8±2.2 |
| K2 | 18.0±1.9 | 17.3±2.5 | 17.6±2.3 | 18.1±2.7 | 17.9±2.3 |
| F | - | - | - | 3.02 | - |
| P | - | - | - | 0.02 | - |
Magnetic resonance findings
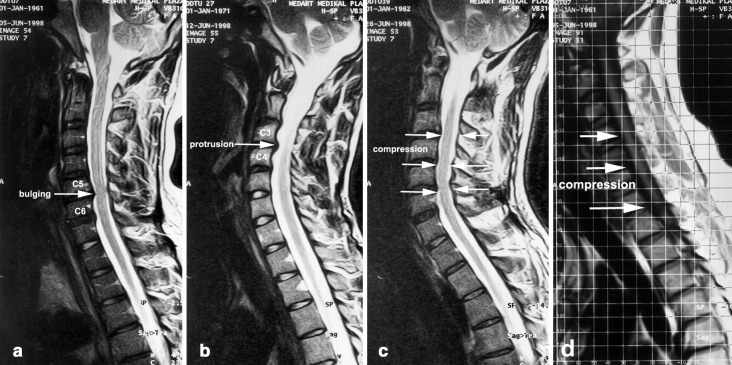
Degenerative changes of the spinal column and the cord were comparatively more severe in the S2 group than in the S1 and K2 groups. Abnormal MR findings of 6 of the 15 subjects in the S1 group, 4 of the 15 subjects in the K1 group, 6 of the 13 subjects in the S2 group, and 7 of the 15 subjects in the K2 group are presented in Table 5. These pathologies included cervical disc bulging (Fig. 5a), osteophytes in the cervical canal, disc protrusion (Fig. 5b), loss of cervical lordosis, spinal cord compression (Fig. 5c), and cord compression in hyperflexion (Fig. 5d).
Table 5.
Magnetic resonance findings of subjects (LCL loss of cervical lordosis, CCHF cord compression in hyperflexion)
| Cervical vertebral column | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | Bulging | Osteophytes | Protrusion | LCL | Cord compression | CCHF | |||||||||
| C3–C4 | C4–C5 | C5–C6 | C6–C7 | C3–C4 | C4–C5 | C5–C6 | C3–C4 | C4–C5 | C5–C6 | - | C3–C4 | C4–C5 | C5–C6 | C6–C7 | |
| S1 | 3 | - | 2 | - | - | - | - | - | - | - | 2 | - | - | 1 | - |
| K1 | 2 | 2 | 1 | 1 | - | - | - | - | - | - | 1 | - | 1 | - | - |
| S2 | 1 | 2 | 2 | 3 | - | 1 | - | - | 1 | 1 | - | - | 2 | 1 | 1 |
| K2 | 1 | 1 | 3 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | - | 3 | 2 | - | - |
Fig. 5.
a Disc bulging at the C5–C6 level of a subject of the S1 group. b Disc protrusion at the C3–C4 level of a subject of the S2 group. c Spinal cord compression of a subject of the S2 group. d Cord compression in hyperflexion in a subject of the S2 group
Two of the subjects in the S1 group had two pathologies simultaneously. These were C5–C6 bulging, and co-existing cord compression at the same level in one subject, and two-level (C3–C4 and C5–C6) disc bulging in the other subject. One subject in the K1 group presented multiple-level (C4–C5, C5–C6 and C6–C7) disc bulging and concomitant spinal cord compression at the C4–C5 level. Only two subjects of the S2 group had single-level (C6–C7) disc bulging. All the other subjects presented multiple-level bulging, protrusion and cord compression. One subject of this group presented spinal cord compression in hyperflexion. Multiple lesions were also common in the K2 group. Transversospinal muscle volumes were not significantly different between the groups (P>0.5; Table 6).
Table 6.
Transversospinal muscle volumes (cm3) of subjects
| Group | n | Semispinalis capitis | Semispinalis cervicis | ||
|---|---|---|---|---|---|
| R | L | R | L | ||
| S1 | 15 | 1.79±0.16 | 1.76±0.24 | 2.00±0.42 | 1.88±0.36 |
| K1 | 13 | 1.78±0.19 | 1.68±0.17 | 1.81±0.30 | 1.78±0.34 |
| S2 | 15 | 1.87±0.19 | 1.92±0.49 | 1.85±0.32 | 1.74±0.27 |
| K2 | 15 | 1.86±0.21 | 1.93±0.40 | 1.91±0.33 | 1.91±0.37 |
Discussion
The head of a soccer player should be rigid at impact to absorb the force of the ball and prevent jarring at the neck. Opposing forces can be considered as an increase in the mass involved at impact by stabilizing the head [19]. When stabilizing the head, the player decreases the risk of rotational acceleration of the head relative to the trunk and also the risk of damaging the brain and the cervical spine.
Timing is an important factor in the correct heading technique, both to ensure that the head is rigid and to achieve maximum use of the body momentum in striking the ball. If the timing is poor, the consequences, in terms of forces involved, will be less. The rigidity of the head is, however, also less and the risk of acceleration of the head is greater.
The 5–7 mm thick scalp is considered as a visco-elastic structure and, like most biological tissues, is a dampening system in which some input energy is dissipated. The skin and the scalp covering of the skull will most probably reduce the impact impulse transferred to the cervical spine via the skull, and prolong the rise time and the duration of the impact. The rise time of acceleration is important for the local effect of the impact and is of no great importance in heading. The increase in contact time is, however, of significance since the force of the ball against the head reduces with the increase in the contact time.
The impact time of the ball to the head in soccer is between 1/64 and 1/125 s. The weight of the ball changes from 396 to 483 g. Air pressure of the ball at sea level is 1 kg/cm2 and the speed of the ball may increase up to 130 km/h during the game. The maximum force applied to the athletes’ head can be calculated as approximately 2,000 N for each heading of the ball from the above given data, and the cervical spine is indirectly influenced by this trauma. The cervical spine absorbs a significant amount of the force generated due to heading the ball. This type of repetitive force during competition or training may increase the risk of degeneration at the intervertebral joints, intervertebral discs or the spinal cord.
Normalized mean extension work and power of the cervical spinal musculature were found to be higher in active soccer players in this study. This increase was not observed in veteran players. The increase in normalized mean extension power in the S1 group did not correlate with the transversospinalis muscle volumes measured in MR. Though the extensor muscles of the cervical spine have been defined as significant dynamic stabilizers [17], our current study showed that their volumes were not significantly different between groups. Isometric cervical extension strength is known to increase with training [12]. The findings of our current study may indicate that the soccer players examined in this study received no special training to increase cervical muscle strength. The effect of such training to decrease trauma and subsequent degeneration is open for further investigation.
Isometric strength measurements of the cervical spine are experimental in nature. Reproducibility [26] and intra- and inter-day reliability [8] of isometric strength measurement of neck muscles have been presented. The testing error of the measurement device developed by Ylinen et al. [26] using two strain gauges was reported as ±1.0 N and ±0.1 Nm with standard loads. Though the testing error of the device of the current study was not measured, the correlation coefficient of repetitive measurement was 0.87, indicating a reasonable coefficient variance.
Jordan et al. [8] demonstrated impressive levels of muscular strength in the flexors and extensors of the cervical spine. Their study reported that cervical muscular strength was maintained until the seventh decade. The normalized mean extension moment, normalized power and normalized work were higher in the S1 group of the current study, indicating a positive effect of playing soccer in the third decade. The higher normalized mean extension moment, normalized power and normalized work of young soccer players, however, was not able to prevent the development of early cervical degenerative changes. Playing soccer in the fourth decade had almost no effect on the isometric strength of the cervical muscles. The overall better muscle fitness condition of soccer players may play a role in the prevention of degenerative changes, and it is assumed that further strengthening of the cervical muscles may ameliorate this aim.
The flexion–extension ROM of the S1 group of this study was lower than that of Ferrario et al. [4] who measured absolute anterior and posterior cervical motion of 30 males with a mean age of 22.8±4.9 as 70.1±15.0 and 69.0±8.8 , respectively. A motion analysis system was used in that study. The ROM in lateral bending and rotation was not measured in our current study. Playing soccer decreased the ROM of the cervical spine over time. This decrease was more significant in active players when compared to their age-matched controls. This finding was in contrast to our common knowledge that ROM of the cervical spine decreases with age [13].
Cervical spinal ROM was measured by active flexion and extension radiographs, but advanced techniques [20, 27] were not used in this study. Intersegmentary angulation was lower in the upper cervical spine of the active soccer players. This was most significant at the C2–C3 level. The intersegmentary angulation of the S2 group was more pronounced than in the K2 group, however, the difference was not significant. Intersegmentary angulation was prominent at the C5–C6 level and decreased with age in both groups. This finding was in agreement with the findings of Dvorak et al. [3] and Mannion et al. [15]. The saggital diameter of the cervical canal decreased in veteran soccer players when compared to active players and their age-matched controls. These findings support our hypothesis and reveal that continuous micro- and macro-trauma to the cervical spine due to heading the ball in soccer may cause early degenerative changes.
Overall MR findings of the spinal column and cord were more severe in soccer players than in their age-matched controls. Cervical disc bulging, osteophytes in the cervical canal, disc protrusion, loss of cervical lordosis, spinal cord compression, and cord compression in hyperflexion were the most common MR findings. These findings strengthen the radiological findings of this study. They reveal that degenerative changes of the cervical spine in soccer are not only limited to the skeletal tissues but may also extend to the soft tissues, including the intervertebral disc and the spinal cord.
In conclusion, biomechanical, radiological, and MR findings present a tendency towards early degenerative changes of the cervical spine most probably due to heading the ball in soccer. These changes tend to increase with age and seem to be irreversible. They are not limited to the skeletal tissue but extend to the intervertebral disc and spinal cord. Patients admitted to a clinic with signs and symptoms of degenerative cervical spine should be evaluated for a history of soccer playing or vice versa. We propose that extensor muscles be strengthened to prevent early degenerative changes to the cervical spine in soccer players, but this needs further investigation.
Acknowledgements
The authors thank Professor Dr. Turgut Tümer and Associate Professor Barış Diren of the Middle East Technical University, Faculty of Engineering, Department of Mechanical Engineering and Medart Medical Center, Kavaklıdere, Ankara, Turkey, for their valuable advice and assistance in developing the cervical dynamometer and obtaining the MR images, respectively. The authors also thank Hülya Aşçı for statistical analysis, Bahadir Kantaroḡlu for the development of the biomechanical dynamometer, and Ahmet Yürekli and Haydar Yaḡcı for their technical assistance in obtaining the cervical radiographs. The Middle East Technical University Research Grant # AFP-98-05-04-02 supported this research.
References
- 1.Berg Arch Phys Med Rehabil. 1994;75:661. doi: 10.1016/0003-9993(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 2.Dihlmann Joints and vertebral. 1985;connections:clinical. [Google Scholar]
- 3.Dvorak J Orthop Res. 1991;9:828. doi: 10.1002/jor.1100090608. [DOI] [PubMed] [Google Scholar]
- 4.Ferrario J Orthop Res. 2002;20:122. doi: 10.1016/S0736-0266(01)00079-1. [DOI] [PubMed] [Google Scholar]
- 5.Fried Sports Med. 1992;14:269. doi: 10.2165/00007256-199214040-00005. [DOI] [PubMed] [Google Scholar]
- 6.Fujiwara K (1998) Prognosis and risk factors in cervical spondylosis. In: Ono K, Dvorak J, Dunn E (eds) Cervical spondylosis and similar disorders. World Scientific, Singapore, pp 627–649
- 7.Hoy Am J Sports Med. 1992;20:318. doi: 10.1177/036354659202000314. [DOI] [PubMed] [Google Scholar]
- 8.JordanSpine 199924134310404577 [Google Scholar]
- 9.Jordan Am J Sports Med. 1996;24:205. doi: 10.1177/036354659602400216. [DOI] [PubMed] [Google Scholar]
- 10.Kalyon TA (1994) Athletes health and sports injuries. GATA, Ankara, 181 pp
- 11.Kurosawa Skeletal Radiol. 1991;20:437. doi: 10.1007/BF00191087. [DOI] [PubMed] [Google Scholar]
- 12.Leggett Am J Sports Med. 1991;19:635. doi: 10.1177/036354659101900618. [DOI] [PubMed] [Google Scholar]
- 13.Lind Arch Phys Med Rehabil. 1989;70:692. [PubMed] [Google Scholar]
- 14.Lindenfeld Am J Sports Med. 1994;22:364. doi: 10.1177/036354659402200312. [DOI] [PubMed] [Google Scholar]
- 15.Mannion Eur Spine J. 2000;9:379. doi: 10.1007/s005860000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matser J Am Med Assoc. 1999;282:971. doi: 10.1001/jama.282.10.971. [DOI] [Google Scholar]
- 17.NolanSpine 19881393381146 [Google Scholar]
- 18.Pollock Arch Phys Med Rehabil. 1993;74:1080. doi: 10.1016/0003-9993(93)90065-i. [DOI] [PubMed] [Google Scholar]
- 19.Reid J Trauma. 1975;15:150. doi: 10.1097/00005373-197502000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Roozmon J Biomed Eng. 1993;15:13. doi: 10.1016/0141-5425(93)90087-f. [DOI] [PubMed] [Google Scholar]
- 21.Scoppetta C, Vaccario ML (1978) Central cervical cord syndrome after heading a football. Lancet 10;1(8076):1269 [DOI] [PubMed]
- 22.Tysvaer Sports Med. 1992;14:200. doi: 10.2165/00007256-199214030-00006. [DOI] [PubMed] [Google Scholar]
- 23.Tysvaer Am J Sports Med. 1991;19:56. doi: 10.1177/036354659101900109. [DOI] [PubMed] [Google Scholar]
- 24.Uslu B (1994) Sports injuries. Headquarter of Youth and Sports Press, Ankara, 34 pp
- 25.White AA, Panjabi MM (1990) Clinical biomechanics of the spine, 2nd edn. Lippincott, Philadelphia, 86 pp
- 26.Ylinen Clin Biomech. 1999;14:217. doi: 10.1016/s0268-0033(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 27.Youdas Phys Ther. 1991;71:98. doi: 10.1093/ptj/71.2.98. [DOI] [PubMed] [Google Scholar]