Abstract
Platelet-rich plasma (PRP) is an autogenous source of growth factor and has been shown to enhance bone healing both in clinical and experimental studies. PRP in combination with porous hydroxyapatite has been shown to increase the bone ingrowth in a bone chamber rat model. The present study investigated whether the combination of beta tricalcium phosphate (β-TCP) and PRP may enhance spinal fusion in a controlled animal study. Ten Danish Landrace pigs were used as a spinal fusion model. Immediately prior to the surgery, 55 ml blood was collected from each pig for processing PRP. Three-level anterior lumbar interbody fusion was performed with carbon fiber cages and staples on each pig. Autogenous bone graft, β-TCP, and β-TCP loaded with PRP were randomly assigned to each level. Pigs were killed at the end of the third month. Fusion was evaluated by radiographs, CT scanning, and histomorphometric analysis. All ten pigs survived the surgery. Platelet concentration increased 4.4-fold after processing. Radiograph examination showed 70% (7/10) fusion rate in the autograft level. All the levels with β-TCP+PRP showed partial fusion, while β-TCP alone levels had six partial fusions and four non-fusions (P=0.08). CT evaluation of fusion rate demonstrated fusion in 50% (5/10) of the autograft levels. Only partial fusion was seen at β-TCP levels and β-TCP+PRP levels. Histomorphometric evaluation found no difference between β-TCP and β-TCP+PRP levels on new bone volume, remaining β-TCP particles, and bone marrow and fibrous tissue volume, while the same parameters differ significantly when compared with autogenous bone graft levels. We concluded from our results in pigs that the PRP of the concentration we used did not improve the bone-forming capacity of β-TCP biomaterial in anterior spine fusion. Both β-TCP and β-TCP+PRP had poorer radiological and histological outcomes than that of autograft after 3 months.
Keywords: Spinal fusion, Bone substitute, Platelet-rich plasma, Tricalcium phosphate, Pig
Introduction
Platelets contain a variety of physiologically active substances. Upon degranulation, such factors as platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), insulin-like growth factors (IGF-1, IGF-II), fibrous growth factor (FGF), and epidermal growth factor (EGF) are released [23]. Platelet-rich plasma (PRP) is an autogenous source of growth factor and has been shown to stimulate osteoblast-like cells in vitro and to enhance bone graft incorporation in maxillofacial applications in vivo [19, 23]. PRP has also been shown to promote early maturation of bony fusion and has yielded good fusion results in lumbar spine fusions clinically [15].
In spinal surgery, the availability of adequate amounts of autogenous bone graft is often limited and is always complicated by the morbidity related to harvesting bone graft from iliac crest [2, 6, 27]. Beta tricalcium phosphate (β-TCP) has been used clinically as a graft extender for autograft and allograft in spinal fusions, and satisfactory results have been reported [10, 14]. However, β-TCP has seldom been used alone in anterior spinal fusion. Among the limited publications describing the use of β-TCP alone in anterior spinal fusion in animal studies, the results have varied [20, 26]. In our previous study, β-TCP provided poorer bone formation in comparison with autograft in an anterior spinal fusion model (H. Li et al. submitted for publication). Composite graft materials comprising osteogenic growth factors and inorganic ceramic carrier have now become an important research direction in an attempt to minimize the use of autograft. PRP in combination with porous hydroxyapatite has been shown to increase bone ingrowth in a bone chamber rat model [22]. In the present study we investigated whether or not the combination of β-TCP and PRP may enhance spinal fusion in a controlled animal study.
Materials and methods
This controlled animal study was carried out on the pig interbody fusion model [12]. Ten Danish Landrace pigs were used and three-level anterior lumbar interbody fusion with carbon fiber cages (Brantigan I/F cage, 8 mm posterior height and 15 mm depth; DePuy AcroMed, Raynham, MA, USA) was performed on each pig. Autogenous bone graft was used as a gold standard control, and β-TCP particles and β-TCP loaded with PRP were used in the other two levels separately. β-TCP was in the form of granules and have the size of 1.5–3.0 mm with 70% porosity (DePuy Bioland, Toulouse, France). The research protocol was approved by the Danish Board for Animal Research.
Immediately prior to surgery, 55 ml blood was collected from each pig in a 60-ml syringe containing 5 ml anticoagulant citrate dextrose-A. An additional blood sample was taken for the determination of hematocrit and baseline platelet count. The 60 ml of anticoagulated blood was processed by means of the Symphony system (DePuy AcroMed) to generate about 7 ml PRP. At the time of surgery, the PRP was mixed with 1 ml thrombin/calcium chloride solution (1,000 U thrombin per milliliter of 10% calcium chloride). A sample of PRP was also used for the determination of platelet count after processing.
Under general anesthesia, cancellous bone graft was taken from the iliac crest, and then the pig was moved into the supine position. With a paramedian incision, the retroperitoneal approach was taken in order to access the anterior lumbar spine. Disc tissue and endplates from three levels (L2/3, L4/5, L6/7) were resected with the posterior longitudinal ligament left intact. The autogenous bone graft, β-TCP, and the β-TCP combined with PRP were separately put into the carbon fiber cages and inserted into different levels. Since β-TCP particles can drop out of the cage upon handling, the prepared disc space was spread slightly to help the insertion process, and only when the cage hole was almost inside the disc space did the hammering start. Each level was secured anteriorly with two staples for fixation. The abdominal wall was carefully sutured. Ampicillin (Anhypen, 1.0 g IV; Gist-Brocades, Delft, The Netherlands) was given pre- and postoperatively, and on the 3 days (1.0 g/day, IM) following the operation. Buprenorphine (Temgesic, Hull, UK; 0.02 mg/kg, IM) was used routinely for 3–5 days to relieve pain.
The pigs were housed individually and fed up to 3 months with controlled diet. Under general anesthesia, the pigs were killed by means of an intravenous injection of an overdose of pentobarbital. The spinal column from L1 to L7 was removed en bloc, stripped of soft tissue, and frozen until examination was conducted.
Radiographs of double projection and CT scanning of sagittal and cross-sectional examination were performed on the specimens. Results were reviewed in a blind fashion. Radiograph and CT informed fusion was defined as a continuous bone bridge across the cage with no obvious interruption by a radiolucent line. Ingrowth of bone into the cage with a radiolucent line in the middle or on the interface was defined as partial fusion. Little or no bone ingrowth into the cage was defined as non-fusion.
Next, the fusion mass was harvested together with the neighboring vertebral bone. They were dehydrated in graded ethanol (70–99%) containing 0.4% basic fuchsin and embedded in PMMA. Serial sagittal sections of 50 μm thick were cut with a Sawing Microtome KDG 95 (Meprotech, Heerhugowaard, The Netherlands). The surface was counterstained with 4% light green for 2 min. Four sections from each specimen were systematically chosen for evaluation. Histological images were captured with a 3-CCD video camera and evaluated in the computer. Blind quantitative evaluations of the slides were performed using the point-counting technique (CAST-Grid software; Olympus, Glostrup, Denmark). Only the area inside the cage was evaluated. Bone volume, bone marrow space, residue β-TCP particles, and fibrous tissue were calculated in percentages.
Data were analyzed by SPSS (version 10.0) and are presented as mean values ± standard deviation (SD). The Q-Q plot (a normality test) for an approximation to normal distribution was utilized. MANOVA for repeated measures was applied to compare the three different treatments. Fusion rates were compared with Fisher’s exact test, and P<0.05 (two-tailed) was considered significant.
Results
All ten pigs survived the surgery with no postoperative complications. Platelet concentration increased 4.4-fold after processing. The blood cell count before and after the centrifugation is shown in Table 1. Upon manual palpation, all of the fusion segments were rigid at the time of killing with the exception of two of the levels in pigs 7 and 8 which showed micromotion, one in the autograft level and another in the β-TCP level. Radiographic examination revealed a 70% (7/10) fusion rate in the autograft level. All of the levels with β-TCP/PRP showed partial fusion, while the exclusively β-TCP levels had six partial fusions and four non-fusions (P=0.08). CT evaluation decreased the fusion rate to 50% (5/10) in autograft levels. β-TCP levels showed the same images of partial fusion on different scanning sections as β-TCP/PRP in all the cases. The results are summarized in Table 2. Remaining particles of β-TCP could be seen from the CT sections, and also faintly seen on radiograph (Fig. 1). Histologically, new bone apposition can be seen next to β-TCP particles. However, cages in β-TCP or β-TCP/PRP levels were largely occupied by fibrous tissue (Fig. 2). Histomorphometric evaluation found no difference between β-TCP and β-TCP/PRP levels with regard to new bone volume, remaining β-TCP particles, and bone marrow and fibrous tissue volume. The same parameters differ significantly when compared with autogenous bone graft levels, which showed a fourfold increase in bone volume fraction (Table 3).
Table 1.
Blood counts before and after processing. (PRP Platelet-rich plasma)
| Platelet | RBC | WBC | |
|---|---|---|---|
| Blood | 398.4×109 | 5.6×1012 | 18.7×109 |
| PRP | 1,786.6×109 | 4.6×1012 | 29.5×109 |
Table 2.
Radiograph and CT evaluation. (β-TCP Beta tricalcium phosphate)
| Graft | X-ray evaluation | CT evaluation | ||||
|---|---|---|---|---|---|---|
| Non-fusion | Partial fusion | Fusion | Non-fusion | Partial fusion | Fusion | |
| β-TCP | 4 | 6 | 0 | 0 | 10 | 0 |
| β-TCP+PRP | 0 | 10 | 0 | 0 | 10 | 0 |
| Autograft | 0 | 3 | 7 | 0 | 5 | 5 |
| P value | P<0.001a | P=0.002a | ||||
aChi-square test (Fisher’s exact test). The difference between β-TCP and β-TCP+PRP were not significant both for X-ray and CT evaluation
Fig. 1a, b.
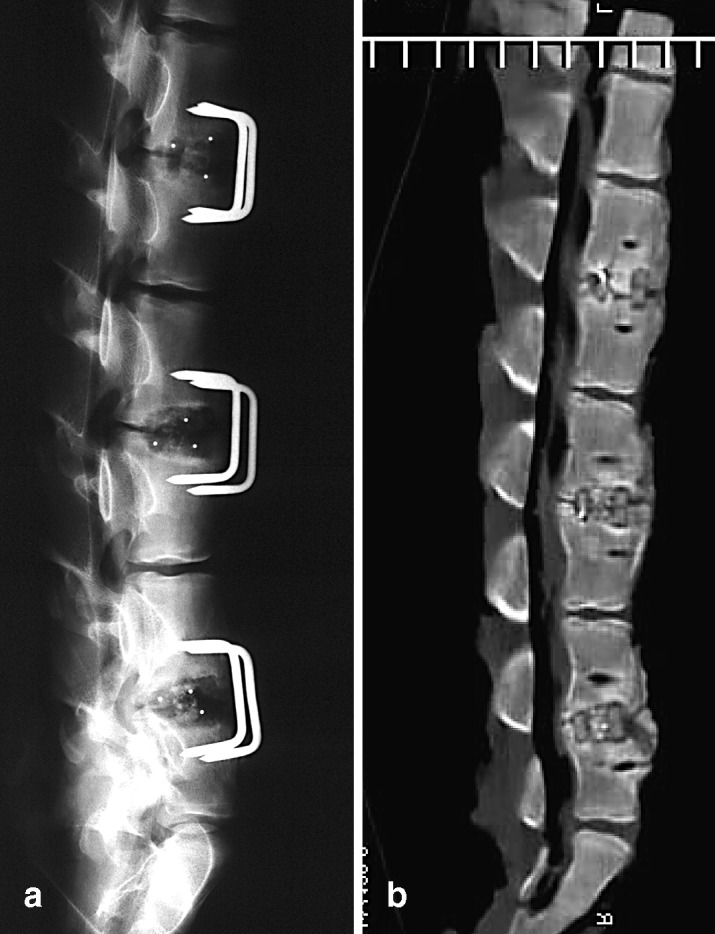
Plain radiograph (a) and CT scan section (b) of the spine of pig 6 after 3 months. L2/3 was graded partial fusion into which autograft was put; beta tricalcium phosphate (β-TCP) was put into L4/5 where the radiograph shows non-fusion and the CT image shows partial fusion; β-TCP loaded with platelet-rich plasma (PRP) was in the lowest level (L6/7) where particles of β-TCP can still been seen
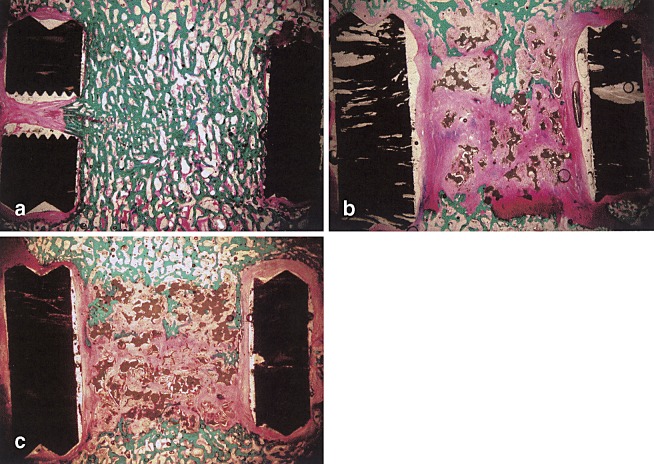
Fig. 2a–c.
Micrographs of histological sections. a Autograft level with typical fusion. b Pure β-TCP level. Bone ingrowth can been seen in the upper middle part. Residue β-TCP particles can still be seen. c β-TCP/PRP level. Limited bone ingrowth as that of pure β-TCP level in b. Magnification approximately 5×1.25
Table 3.
Histomorphometric evaluations. Data are presented as percentage of the total volume (mean±SD). The total sum is lower than 100% in each column because the resorption space around β-TCP and the shrinkage space near the cage are not listed
| β-TCP | β-TCP/PRP | Autograft | |
|---|---|---|---|
| Total bone | 11.43±6.53 | 9.50±4.32 | 42.75±10.56* |
| Bone marrow | 7.48±4.78 | 6.29±3.56 | 31.40±5.49* |
| Cartilage | 2.09±2.45 | 1.65±2.46 | 6.84±4.43** |
| Residual β-TCP | 13.62±6.03 | 13.54±8.79 | 0 |
| Fibrous tissue | 57.32±11.30 | 57.70±10.8 | 15.13±13.52* |
*P<0.001 (ANOVA)
**P<0.05 (ANOVA)
Discussion
Platelet-rich plasma has the advantage of being autologous, endogenously derived, and a natural growth factor that is easy to apply and poses no risk for transmittable diseases. However, our present study showed that addition of PRP into the β-TCP-loaded cages did not increase bone formation or alter β-TCP resorption in this particular mode; cages with β-TCP had significantly less new bone formation than those with autograft.
A porcine model of three-level lumbar interbody fusion was employed in this study. The porcine model was described in our previous studies and had been shown to be a feasible model when human implants are to be engaged [12, 29]. In the present experiment, the pigs had a mean serum platelet concentration of 398,400/μl, which is slightly higher than that among humans. After our processing, platelets reached a concentration of 1.7×1012/ml in a total of 7 ml plasma, which is 4.4 times the serum level. This concentration is comparable to the effective concentration of 1.0×1012/ml reported in the literature [18]. The specific growth factors in the PRP were not quantified in the present study, but the growth factors contained inside the porcine platelets have been reported in the literature [8, 21]. Due to the pigs’ low hematocrit levels, we performed additional procedures by hand with a syringe in order to collect the buffy coat. This yielded a 1.6 times higher concentration of white blood cells than that in the serum. We know that white blood cells are responsible for inflammatory reaction in the early stage of fracture healing. The exact influence of higher white blood cell concentration on the β-TCP in the cage in our study is unclear, but in the literature, suppression of leukocyte proliferation or function resulted in higher bone formation and mechanical bending moment in rats [9, 28].
Based on the fact that ceramic alone is less effective in comparison to autograft in interbody fusion [5, 7], the combination of ceramic and growth factors is now under vigorous investigation. In the present experimental design, β-TCP functions both as an osteoconductive scaffold and a carrier for PRP. Growth factors released from platelets should have an intact bioactivity. Besides PDGF, platelets are also the richest source of TGF-β other than bone tissue. Both PDGF and TGF-β have been shown to promote osteogenesis in vivo [11, 13]. However, the effects of PDGF alone have been inconsistent; local administration of PDGF failed to achieve significant increase in bone ingrowth into HA carriers in rat femora [1] and PDGF even inhibits bone regeneration induced by osteogenin in rat calvarial defects [16]. Other factors released by platelets, such as EGF and IGF, also demonstrate positive effects on osteoblast or bone formation [17, 24]. The overall effect of platelet concentrate on the stimulation of osteoblast proliferation in vitro and enhancement of bone graft incorporation in maxillofacial application has been reported [19, 23]. In a bone chamber rat model, platelet concentrate can increase the bone ingrowth into the porous hydroxyapatite [22]. We could not find the anticipated effect of PRP in the cages filled with β-TCP in our pig model. Possible reasons include:
The optimal amount of PRP needed in this big animal model. Though we produced about 7 ml PRP, only about 2–3 ml was enough to saturate the β-TCP particles inside the cage. The remaining PRP was discarded. Those 2–3 ml PRP may not be enough to generate a detectable effect. On the other hand, the exact amount and forms of PDGF and TGF-β presented in the pig platelet were unknown. In a similar study of growth factor and β-TCP in vertebral body defect-filling in a baboon model [26], TGF-β3 did not show an enhancement of bone incorporation with β-TCP.
The biological environment of anterior spinal fusion inside the cage is quite different from that of posterior fusion. Ceramics yielded generally good results in posterior lateral fusion models [3, 25]. Though we used a fenestrated cage, vessel and fibrous infiltration enter mainly from both ends, unlike the situation in the posterior lateral fusion where vessel infiltration and cell recruitment occur more easily. Even with posterior lateral fusion alone, there is a significant difference of ceramic incorporation between laminar and intertransverse sites [4].
Micromotion of the studied segments. We found that fibrous or fibrocartilaginous lines were frequently seen in the cage/vertebral interface. However, as we randomized the levels, so the influence of micromotion should affect all the levels equally.
The effect of PRP is generally considered to be mitogenesis, angiogenesis, and chemotaxis. An early effect of the PRP was therefore expected at the start of the experiment, and the observation time was set at 3 months, during which a reasonable fusion rate for autograft levels has been observed in our previous study [12]. Based on the later histomorphometric evaluation, there remain about 14% unabsorbed β-TCP granules. These residual β-TCP granules, hardly discernable on plain radiographs, can be visualized on CT images. So the CT evaluation results were the same for β-TCP and β-TCP/PRP levels. The absence of one group with a longer observation time limits us from further conclusions. Histomorphometrically, cages filled with β-TCP were largely occupied by fibrous tissue. Possible explanations could be that small granules were easily absorbed, and the problem of filling the cage properly may also lead to excessive fibrous formation. Calculation of the absorption rate of β-TCP particles was also unattainable, because the initial filling volume was unknown. A cage with prefilled β-TCP structure could be a good solution.
We concluded that autogenous bone graft achieves the best results when compared with β-TCP particles in our spinal fusion model with cages. Application of PRP of the aforementioned concentration into the β-TCP particles did not demonstrate any effect on bone formation or β-TCP resorption in this study.
Acknowledgements
The study was supported by Depuy AcroMed, Inc. Raynham, MA, USA
References
- 1.Arm Biomaterials. 1996;17:703. doi: 10.1016/0142-9612(96)86740-8. [DOI] [PubMed] [Google Scholar]
- 2.Banwart Spine. 1995;20:1055. doi: 10.1097/00007632-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Boden Spine. 1999;24:1179. doi: 10.1097/00007632-199906150-00002. [DOI] [PubMed] [Google Scholar]
- 4.Delecrin Spine. 1997;22:1683. doi: 10.1097/00007632-199708010-00001. [DOI] [PubMed] [Google Scholar]
- 5.Emery Spine. 1996;21:2713. doi: 10.1097/00007632-199612010-00003. [DOI] [PubMed] [Google Scholar]
- 6.Fernyhough Spine. 1992;17:1474. doi: 10.1097/00007632-199212000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Fuller Spine. 1996;21:2131. doi: 10.1097/00007632-199609150-00015. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Bolao Eur J Clin Invest. 1996;26:929. doi: 10.1111/j.1365-2362.1996.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 9.Grogaard Arch Orthop Trauma Surg. 1990;109:268. doi: 10.1007/BF00419942. [DOI] [PubMed] [Google Scholar]
- 10.Gunzburg Orthopedics. 2002;25:s591. doi: 10.3928/0147-7447-20020502-08. [DOI] [PubMed] [Google Scholar]
- 11.LeeJ Periodontol 20007141810776929 [Google Scholar]
- 12.Li Eur Spine J. 2002;11:476. doi: 10.1007/s00586-002-0455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lind Acta Orthop Scand. 1993;64:553. doi: 10.3109/17453679308993691. [DOI] [PubMed] [Google Scholar]
- 14.Linovitz Orthopedics. 2002;25:s585. doi: 10.3928/0147-7447-20020502-07. [DOI] [PubMed] [Google Scholar]
- 15.Lowery Bone. 1999;25:47S. doi: 10.1016/S8756-3282(99)00132-5. [DOI] [PubMed] [Google Scholar]
- 16.Marden J Clin Invest. 1993;92:2897. doi: 10.1172/JCI116912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marie Am J Physiol. 1990;258:E275. doi: 10.1152/ajpendo.1990.258.2.E275. [DOI] [PubMed] [Google Scholar]
- 18.Marx Implant Dent. 2001;10:225. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Marx Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 20.Ohyama J Neurosurg. 2002;97:350. doi: 10.3171/spi.2002.97.3.0350. [DOI] [PubMed] [Google Scholar]
- 21.Olutoye J Pediatr Surg. 1997;32:827. doi: 10.1016/s0022-3468(97)90629-1. [DOI] [PubMed] [Google Scholar]
- 22.SiebrechtOrthopedics 20022516911871379 [Google Scholar]
- 23.SlaterJ Orthop Res 1995136557472743 [Google Scholar]
- 24.SpencerBone 199112212054232 [Google Scholar]
- 25.Steffen Clin Orthop. 2000;371:28. doi: 10.1097/00003086-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Steffen Eur Spine J. 2001;10:S132. doi: 10.1007/s005860100325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Summers J Bone Joint Surg Br. 1989;71:677. doi: 10.1302/0301-620X.71B4.2768321. [DOI] [PubMed] [Google Scholar]
- 28.Voggenreiter J Bone Miner Res. 2000;15:1825. doi: 10.1359/jbmr.2000.15.9.1825. [DOI] [PubMed] [Google Scholar]
- 29.Zou Acta Orthop Scand. 2003;74:596. doi: 10.1080/00016470310018027. [DOI] [PubMed] [Google Scholar]