Abstract
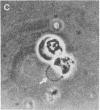
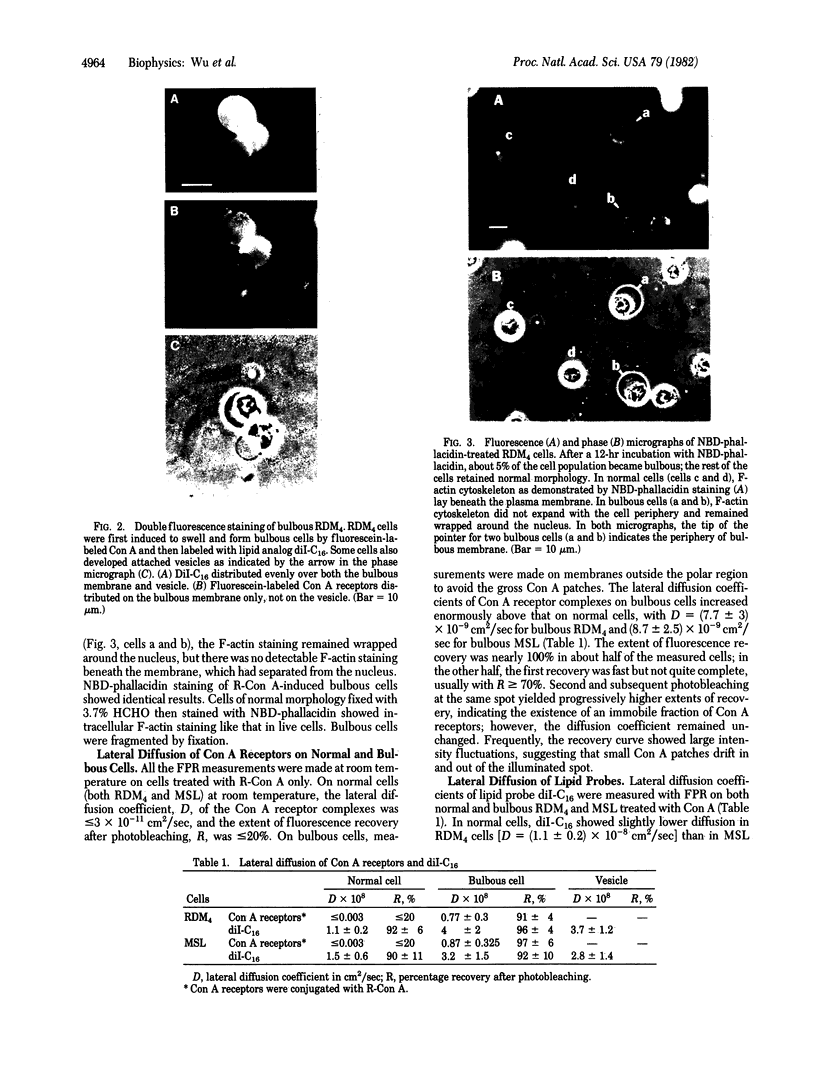
The lateral diffusion coefficient, D, of concanavalin A receptors and receptor complexes on the surface of lymphocytes and RDM4 lymphomas is enhanced by several orders of magnitude to D greater than 5 X 10(-9)cm2/sec by induction of swelling of the cells to bulbous form. Treatments with concanavalin A or 7-nitrobenz-2-oxa-1,3-diazole-phallacidin induce blebs and the bulbous form. The resulting separation of the plasma membrane from most of the F-actin cytoskeleton is accompanied by release of constraints on lateral diffusion of the cell surface receptors, which allows the diffusivity of these glycoproteins to increase nearly to the limit allowed by membrane viscosity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ash J. F., Singer S. J. Concanavalin-A-induced transmembrane linkage of concanavalin A surface receptors to intracellular myosin-containing filaments. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4575–4579. doi: 10.1073/pnas.73.12.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak L. S., Yocum R. R., Nothnagel E. A., Webb W. W. Fluorescence staining of the actin cytoskeleton in living cells with 7-nitrobenz-2-oxa-1,3-diazole-phallacidin. Proc Natl Acad Sci U S A. 1980 Feb;77(2):980–984. doi: 10.1073/pnas.77.2.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak L. S., Yocum R. R., Webb W. W. In vivo staining of cytoskeletal actin by autointernalization of nontoxic concentrations of nitrobenzoxadiazole-phallacidin. J Cell Biol. 1981 May;89(2):368–372. doi: 10.1083/jcb.89.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S. Directed lipid flow in cell membranes. Nature. 1976 Mar 4;260(5546):21–23. doi: 10.1038/260021a0. [DOI] [PubMed] [Google Scholar]
- Cherry R. J. Rotational and lateral diffusion of membrane proteins. Biochim Biophys Acta. 1979 Dec 20;559(4):289–327. doi: 10.1016/0304-4157(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Dragsten P., Henkart P., Blumenthal R., Weinstein J., Schlessinger J. Lateral diffusion of surface immunoglobulin, Thy-1 antigen, and a lipid probe in lymphocyte plasma membranes. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5163–5167. doi: 10.1073/pnas.76.10.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Edidin M. Rotational and translational diffusion in membranes. Annu Rev Biophys Bioeng. 1974;3(0):179–201. doi: 10.1146/annurev.bb.03.060174.001143. [DOI] [PubMed] [Google Scholar]
- Fahey P. F., Webb W. W. Lateral diffusion in phospholipid bilayer membranes and multilamellar liquid crystals. Biochemistry. 1978 Jul 25;17(15):3046–3053. doi: 10.1021/bi00608a016. [DOI] [PubMed] [Google Scholar]
- Flanagan J., Koch G. L. Cross-linked surface Ig attaches to actin. Nature. 1978 May 25;273(5660):278–281. doi: 10.1038/273278a0. [DOI] [PubMed] [Google Scholar]
- Golan D. E., Veatch W. Lateral mobility of band 3 in the human erythrocyte membrane studied by fluorescence photobleaching recovery: evidence for control by cytoskeletal interactions. Proc Natl Acad Sci U S A. 1980 May;77(5):2537–2541. doi: 10.1073/pnas.77.5.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty D. L., Hay E. D. Freeze-fracture studies of the developing cell surface. II. Particle-free membrane blisters on glutaraldehyde-fixed corneal fibroblasts are artefacts. J Cell Biol. 1978 Sep;78(3):756–768. doi: 10.1083/jcb.78.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henis Y. I., Elson E. L. Inhibition of the mobility of mouse lymphocyte surface immunoglobulins by locally bound concanavalin A. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1072–1076. doi: 10.1073/pnas.78.2.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K., Derzko Z., Wu E. S., Hou Y., Poste G. Measurement of the lateral mobility of cell surface components in single, living cells by fluorescence recovery after photobleaching. J Supramol Struct. 1976;5(4):565(417)–576(428). doi: 10.1002/jss.400050411. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Hou Y., Derzko Z., Wojcieszyn J., Organisciak D. Lipid lateral diffusion in the surface membrane of cells and in multibilayers formed from plasma membrane lipids. Biochemistry. 1981 Sep 1;20(18):5268–5275. doi: 10.1021/bi00521a027. [DOI] [PubMed] [Google Scholar]
- Koppel D. E., Sheetz M. P., Schindler M. Matrix control of protein diffusion in biological membranes. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3576–3580. doi: 10.1073/pnas.78.6.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A., Entine G. Lateral diffusion of visual pigment in photorecptor disk membranes. Science. 1974 Aug 2;185(4149):457–459. doi: 10.1126/science.185.4149.457. [DOI] [PubMed] [Google Scholar]
- Magde D., Elson E. L., Webb W. W. Fluorescence correlation spectroscopy. II. An experimental realization. Biopolymers. 1974 Jan;13(1):29–61. doi: 10.1002/bip.1974.360130103. [DOI] [PubMed] [Google Scholar]
- Mescher M. F., Jose M. J., Balk S. P. Actin-containing matrix associated with the plasma membrane of murine tumour and lymphoid cells. Nature. 1981 Jan 15;289(5794):139–144. doi: 10.1038/289139a0. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Transmembrane control of the receptors on normal and tumor cells. I. Cytoplasmic influence over surface components. Biochim Biophys Acta. 1976 Apr 13;457(1):57–108. doi: 10.1016/0304-4157(76)90014-9. [DOI] [PubMed] [Google Scholar]
- Poo M., Cone R. A. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974 Feb 15;247(5441):438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- Roath S., Newell D., Polliack A., Alexander E., Lin P. S. Scanning electron microscopy and the surface morphology of human lymphocytes. Nature. 1978 May 4;273(5657):15–18. doi: 10.1038/273015a0. [DOI] [PubMed] [Google Scholar]
- Rotman B., Papermaster B. W. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc Natl Acad Sci U S A. 1966 Jan;55(1):134–141. doi: 10.1073/pnas.55.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein J. L., Smith B. A., McConnell H. M. Lateral diffusion in binary mixtures of cholesterol and phosphatidylcholines. Proc Natl Acad Sci U S A. 1979 Jan;76(1):15–18. doi: 10.1073/pnas.76.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffman P. G., Delbrück M. Brownian motion in biological membranes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3111–3113. doi: 10.1073/pnas.72.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler M., Koppel D. E., Sheetz M. P. Modulation of membrane protein lateral mobility by polyphosphates and polyamines. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1457–1461. doi: 10.1073/pnas.77.3.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Axelrod D., Koppel D. E., Webb W. W., Elson E. L. Lateral transport of a lipid probe and labeled proteins on a cell membrane. Science. 1977 Jan 21;195(4275):307–309. doi: 10.1126/science.556653. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Elson E. L., Webb W. W., Yahara I., Rutishauser U., Edelman G. M. Receptor diffusion on cell surfaces modulated by locally bound concanavalin A. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1110–1114. doi: 10.1073/pnas.74.3.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Koppel D. E., Axelrod D., Jacobson K., Webb W. W., Elson E. L. Lateral transport on cell membranes: mobility of concanavalin A receptors on myoblasts. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2409–2413. doi: 10.1073/pnas.73.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Webb W. W., Elson E. L., Metzger H. Lateral motion and valence of Fc receptors on rat peritoneal mast cells. Nature. 1976 Dec 9;264(5586):550–552. doi: 10.1038/264550a0. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Schindler M., Koppel D. E. Lateral mobility of integral membrane proteins is increased in spherocytic erythrocytes. Nature. 1980 Jun 12;285(5765):510–511. doi: 10.1038/285510a0. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Smith L. M., Smith B. A., McConnell H. M. Lateral diffusion of M-13 coat protein in model membranes. Biochemistry. 1979 May 29;18(11):2256–2259. doi: 10.1021/bi00578a019. [DOI] [PubMed] [Google Scholar]
- Tank D. W., Wu E. S., Webb W. W. Enhanced molecular diffusibility in muscle membrane blebs: release of lateral constraints. J Cell Biol. 1982 Jan;92(1):207–212. doi: 10.1083/jcb.92.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz W. L., Jacobson K., Wu E. S., Derzko Z. Lateral mobility of an amphipathic apolipoprotein, ApoC-III, bound to phosphatidylcholine bilayers with and without cholesterol. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5645–5649. doi: 10.1073/pnas.76.11.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb W. W., Barak L. S., Tank D. W., Wu E. S. Molecular mobility on the cell surface. Biochem Soc Symp. 1981;(46):191–205. [PubMed] [Google Scholar]
- Wolf D. E., Handyside A. H., Edidin M. Effect of microvilli on lateral diffusion measurements made by the fluorescence photobleaching recovery technique. Biophys J. 1982 Jun;38(3):295–297. doi: 10.1016/S0006-3495(82)84561-X. [DOI] [PMC free article] [PubMed] [Google Scholar]